Generation of monoclonal antibody fragments binding the native γ-secretase complex for use in structural studies.
Jean-René Alattia, Claude Schweizer, Matthias Cacquevel, Mitko Dimitrov, Lorène Aeschbach, Mustapha Oulad-Abdelghani, Patrick C Fraering
Index: Biochemistry 51(44) , 8779-90, (2012)
Full Text: HTML
Abstract
A detailed understanding of γ-secretase structure is crucially needed to elucidate its unique properties of intramembrane protein cleavage and to design therapeutic compounds for the safe treatment of Alzheimer's disease. γ-Secretase is an enzyme complex composed of four membrane proteins, and the scarcity of its supply associated with the challenges of crystallizing membrane proteins is a major hurdle for the determination of its high-resolution structure. This study addresses some of these issues, first by adapting CHO cells overexpressing γ-secretase to growth in suspension, thus yielding multiliter cultures and milligram quantities of highly purified, active γ-secretase. Next, the amounts of γ-secretase were sufficient for immunization of mice and allowed generation of Nicastrin- and Aph-1-specific monoclonal antibodies, from which Fab fragments were proteolytically prepared and subsequently purified. The amounts of γ-secretase produced are compatible with robot-assisted crystallogenesis using nanoliter technologies. In addition, our Fab fragments bind exposed regions of native γ-secretase in a dose-dependent manner without interfering with its catalytic properties and can therefore be used as specific tools to facilitate crystal formation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
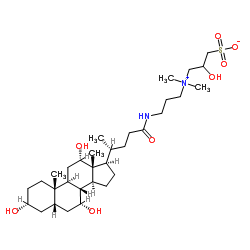 |
CHAPSO
CAS:82473-24-3 |
C32H58N2O8S |
|
Capillary electrophoresis with laser-induced fluorescence de...
2015-01-01 [Talanta 131 , 366-71, (2014)] |
|
Partial loss of the DNA repair scaffolding protein, Xrcc1, r...
2015-07-01 [Neurobiol. Aging 36 , 2319-30, (2015)] |
|
3-O-alkyl-D-glucose derivatives induce fruit bodies of Pleur...
2005-03-01 [Mycol. Res. 109(Pt 3) , 374-6, (2005)] |
|
Detergent-solubilized bovine cytochrome c oxidase: dimerizat...
2000-10-24 [Biochemistry 39 , 12996-13004, (2000)] |
|
Rat brain gamma-secretase activity is highly influenced by d...
2007-06-26 [Biochemistry 46(25) , 7647-54, (2007)] |