Aspergillus fumigatus CY018, an endophytic fungus in Cynodon dactylon as a versatile producer of new and bioactive metabolites.
J Y Liu, Y C Song, Z Zhang, L Wang, Z J Guo, W X Zou, R X Tan
Index: J. Biotechnol. 114(3) , 279-87, (2004)
Full Text: HTML
Abstract
Aspergillus fumigatus CY018 was recognized as an endophytic fungus for the first time in the leaf of Cynodon dactylon. By bioassay-guided fractionation, the EtOAc extract of a solid-matrix steady culture of this fungus afforded two new metabolites, named asperfumoid (1) and asperfumin (2), together with six known bioactive compounds including monomethylsulochrin, fumigaclavine C, fumitremorgin C, physcion, helvolic acid and 5alpha,8alpha-epidioxy-ergosta-6,22-diene-3beta-ol as well as other four known compounds ergosta-4,22-diene-3beta-ol, ergosterol, cyclo(Ala-Leu) and cyclo(Ala-Ile). Through detailed spectroscopic analyses including HRESI-MS, homo- and hetero-nuclear correlation NMR experiments (HMQC, COSY, NOESY and HMBC), the structures of asperfumoid and asperfumin were established to be spiro-(3-hydroxyl-2,6-dimethoxyl-2,5-diene-4-cyclohexone-(1,3')-5'-methoxyl-7'-methyl-(1'H, 2'H, 4'H)-quinoline-2',4'-dione) and 5-hydroxyl-2-(6-hydroxyl-2-methoxyl-4-methylbenzoyl)-3,6-dimethoxyl-benzoic methyl ester, respectively. All of the 12 isolates were subjected to in vitro bioactive assays against three human pathogenic fungi Candida albicans, Tricophyton rubrum and Aspergillus niger. As a result, asperfumoid, fumigaclavine C, fumitremorgin C, physcion and helvolic acid were shown to inhibit C. albicans with MICs of 75.0, 31.5, 62.5, 125.0 and 31.5 microg/mL, respectively.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
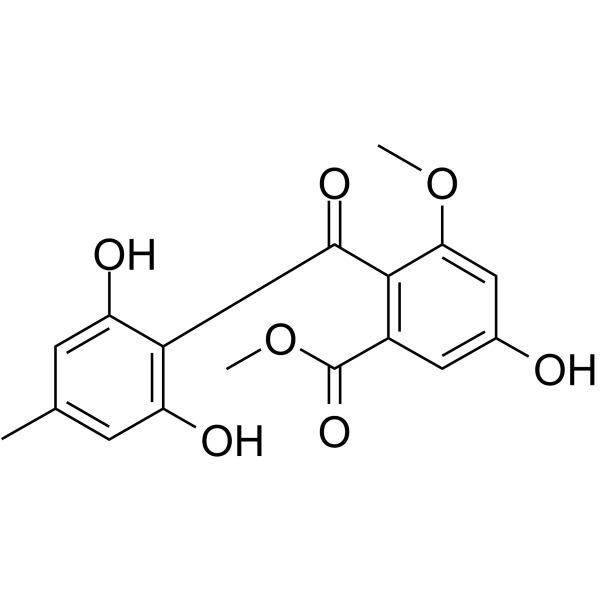 |
Sulochrin
CAS:519-57-3 |
C17H16O7 |
|
New chlorinated diphenyl ethers from an Aspergillus species.
2002-01-01 [J. Nat. Prod. 65(1) , 7-10, (2002)] |
|
Physiological, morphological and kinetic aspects of lovastat...
2009-05-01 [Biotechnol. J. 4(5) , 647-64, (2009)] |
|
A liquid chromatography/tandem mass spectrometric multi-myco...
2007-11-01 [Anal. Bioanal. Chem 389(5) , 1505-23, (2007)] |
|
Sulochrin inhibits eosinophil degranulation.
1997-11-01 [J. Antibiot. 50(11) , 972-4, (1997)] |
|
Effects of ortho-substituent groups of sulochrin on inhibito...
1999-07-19 [Bioorg. Med. Chem. Lett. 9(14) , 1945-8, (1999)] |