C-C bond formation at C-2 of a quinoline ring: synthesis of 2-(1H-indol-3-yl)quinoline-3-carbonitrile derivatives as a new class of PDE4 inhibitors.
K Shiva Kumar, S Kiran Kumar, B Yogi Sreenivas, Dhilli Rao Gorja, Ravikumar Kapavarapu, D Rambabu, G Rama Krishna, C Malla Reddy, M V Basaveswara Rao, Kishore V L Parsa, Manojit Pal
Index: Bioorg. Med. Chem. 20(7) , 2199-207, (2012)
Full Text: HTML
Abstract
A number of 2-(1H-indol-3-yl)quinoline-3-carbonitrile derivatives were synthesized via AlCl(3)-mediated C-C bond forming reaction between 2-chloroquinoline-3-carbonitrile and various indoles. The methodology does not require any N-protection of the indoles employed and provided the corresponding products in good yields. The molecular structure of a representative compound was established unambiguously by single crystal X-ray diffraction and structural elaboration of a compound synthesized has been demonstrated. Many of these compounds synthesized showed PDE4 inhibitory properties in vitro. A brief structure-activity relationship studies within the series along with docking results of a representative compound (EC(50) ∼0.89 μM) is presented.Copyright © 2012 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
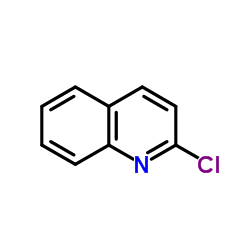 |
Chloroquinoline
CAS:612-62-4 |
C9H6ClN |
|
Antiprotozoal activity of chloroquinoline based chalcones.
2011-05-01 [Eur. J. Med. Chem. 46 , 1897-905, (2011)] |
|
Ruthenium-catalyzed cyclization of anilides with substituted...
2014-07-03 [Org. Lett. 16(13) , 3568-71, (2014)] |
|
Chemoenzymatic synthesis of chiral 2,2'-bipyridine ligands a...
2010-03-07 [Org. Biomol. Chem. 8(5) , 1081-90, (2010)] |
|
Discovering some novel 7-chloroquinolines carrying a biologi...
2013-01-01 [Chem. Pharm. Bull. 61(1) , 50-8, (2013)] |
|
A physico-chemical and biological study of novel chitosan-ch...
2011-10-01 [Int. J. Biol. Macromol. 49(3) , 356-61, (2011)] |