Interaction of CJZ3, a lomerizine derivative, with ATPase activity of human P-glycoprotein in doxorubicin-resistant human myelogenous leukemia (K562/DOX) cells.
Bian-Sheng Ji, Ming Li, Ling He
Index: Pharmazie 65(7) , 515-9, (2010)
Full Text: HTML
Abstract
P-Glycoprotein, a 170-180 kDa membrane glycoprotein that mediates multidrug resistance, hydrolyses ATP to efflux a broad spectrum of hydrophobic agents. To observe the interaction of a P-gp reversal agent with P-gp ATPase activity should provide further insights into the mechanisms of P-gp modulator. In this study, we analysed the effect of CJZ3, a lomerizine derivative, on the adenosine triphosphatase (ATPase) activity of human P-glycoprotein. The results showed that the basal P-gp ATPase activity was increased by CJZ3 with half-maximal activity concentration (Km) of 6.8 +/- 1.5 microM, CJZ3 may interact with P-gp with a higher affinity and exhibit a more potent effect than verapamil (Ver). Kinetic analysis indicated a noncompetitive inhibition of Ver-stimulated P-gp ATPase activity and a competitive inhibition of CJX2-stimulated P-gp ATPase activity by CJZ3, moreover, the effect of CsA on CJZ3-stimulated and Ver-stimulated P-gp ATPase activity showed a non-competitive and a competitive inhibition respectively. CJZ3 and CJX2 can bind P-gp either on overlapping sites or distinct but interacting sites, while CJZ3 and Ver as well as CsA can bind P-gp on separated sites in K562/DOX cells.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
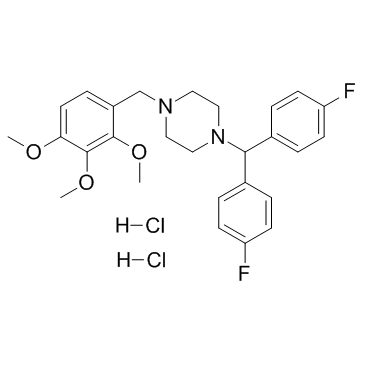 |
Lomerizine hydrochloride
CAS:101477-54-7 |
C27H32Cl2F2N2O3 |
|
A screen of approved drugs and molecular probes identifies t...
2015-06-03 [Sci. Transl. Med. 7 , 290ra89, (2015)] |
|
Neuroprotection by lomerizine, a prophylactic drug for migra...
2011-12-01 [Mol. Cell Biochem. 358(1-2) , 1-11, (2011)] |
|
Goshuyuto, a traditional Japanese medicine for migraine, inh...
2008-09-01 [J. Pharmacol. Sci. 108(1) , 89-94, (2008)] |
|
CJZ3, a lomerizine derivative, modulates P-glycoprotein func...
2006-04-01 [Acta Pharmacol. Sin. 27(4) , 414-8, (2006)] |
|
A new calcium channel antagonist, lomerizine, alleviates sec...
2006-03-01 [Curr. Eye Res. 31(3) , 273-83, (2006)] |