Recombinant expression and purification of human androgen receptor in a baculovirus system.
Z Zhu, O V Bulgakov, S S Scott, J T Dalton
Index: Biochem. Biophys. Res. Commun. 284(3) , 828-35, (2001)
Full Text: HTML
Abstract
A full-length human androgen receptor (hAR) cDNA was used to produce recombinant baculovirus. Spodoptera frugiperda (Sf9) cells infected with this virus expressed protein with an N-terminal hexahistidine tag (His(6)-hAR) in soluble and insoluble forms. The soluble cytosolic His(6)-hAR demonstrated similar association and dissociation half-times for mibolerone, similar binding affinity for mibolerone, and similar steroid specificity as bona fide AR. Under native conditions, the soluble cytosolic His(6)-hAR was purified to apparent homogeneity in the presence of dihydrotestosterone, using metal ion affinity chromatography. The insoluble pellet fraction was solubilized with strong denaturant 6 M guanidine HCl, and His(6)-hAR was purified from it in the presence of 6 M guanidine HCl. Both the solubilized crude pellet fraction and the solubilized/purified His(6)-hAR could be renatured to bind mibolerone. The baculovirus system will therefore provide an efficient means for producing hAR for ligand-binding assays, as well as purifying hAR for detailed molecular analyses.Copyright 2001 Academic Press.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
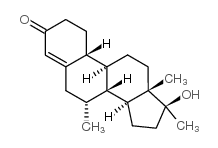 |
Mibolerone
CAS:3704-09-4 |
C20H30O2 |
|
In vivo and in vitro effect of novel 4,16-pregnadiene-6,20-d...
2008-09-01 [J. Steroid Biochem. Mol. Biol. 111(3-5) , 275-81, (2008)] |
|
Steroid and beta-adrenergic receptor modifications in target...
2008-06-01 [Food Chem. Toxicol. 46(6) , 2239-43, (2008)] |
|
Steroids with a carbamate function at C-17, a novel class of...
2007-10-01 [J. Steroid Biochem. Mol. Biol. 107(1-2) , 48-56, (2007)] |
|
Perillyl alcohol inhibits the expression and function of the...
2006-05-18 [Cancer Lett. 236(2) , 222-8, (2006)] |
|
The F-box protein SKP2 mediates androgen control of p27 stab...
2002-08-20 [BMC Cell Biol. 3 , 22, (2002)] |