Separation and quantitation of Z-isomer in lanoconazole by normal phase HPLC.
A Phani Kumar, V R L Ganesh, D V Subba Rao, C Anil, V S Hariharakrishnan
Index: J. Pharm. Biomed. Anal. 50(3) , 535-7, (2009)
Full Text: HTML
Abstract
An isocratic normal phase high-performance liquid chromatographic (NP-HPLC) method has been developed and validated for the quantitation of Z-isomer in lanoconazole. Separation was achieved with a Thermo Hypersil Silica column. The ratio of 2-propanol, n-hexane and triethylamine in the mobile phase were optimized to obtain the best separation. UV detection was performed at 296 nm. The described method is linear over a range of LOQ--15.0 microg/ml of Z-isomer. The mean recovery of Z-isomer was found to be in the range of 97-99%. The method is simple, rapid, selective, accurate and precise, useful in the quality control of bulk manufacturing.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
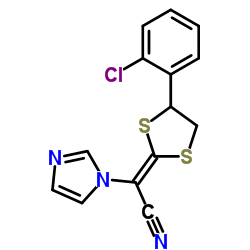 |
Astat
CAS:101530-10-3 |
C14H10ClN3S2 |
|
Allergic contact dermatitis due to both lanoconazole and net...
2001-01-01 [Contact Dermatitis 44(1) , 48-9, (2001)] |
|
Allergic contact dermatitis from diethyl sebacate in lanocon...
2000-10-01 [Contact Dermatitis 43(4) , 233-4, (2000)] |
|
Allergic contact dermatitis from lanoconazole.
1996-07-01 [Contact Dermatitis 35(1) , 63, (1996)] |
|
Synergy of lysozyme and lanoconazole on the morphology of Ca...
2001-01-01 [J. Electron Microsc. (Tokyo) 50(1) , 41-9, (2001)] |
|
Development of a new medium useful for the recovery of derma...
2002-01-01 [Microbiol. Immunol. 46(2) , 83-8, (2002)] |