| Structure | Name/CAS No. | Articles |
|---|---|---|
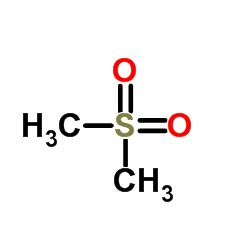 |
Dimethyl sulfone
CAS:67-71-0 |
|
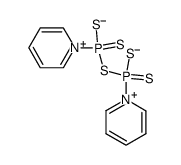 |
P4S10-Pyridine complex
CAS:16610-51-8 |