Direct injection HPLC method for the determination of phenylbutazone and oxyphenylbutazone in serum using a semipermeable surface column.
A Haque, J T Stewart
Index: J. Pharm. Biomed. Anal. 16(2) , 287-93, (1997)
Full Text: HTML
Abstract
A direct injection HPLC method has been developed for the determination of phenylbutazone and its active metabolite oxyphenylbutazone in serum using a semipermeable surface (SPS) column. The method is easy to perform and requires 20 microliters of a filtered serum sample. The chromatographic time is less than 13 min using a mobile phase of 15:85 v/v acetonitrile-0.05M phosphate buffer pH 7.5. The method was linear in the range 0.5-20 micrograms ml-1 (r > 0.99, n = 6) with R.S.D. less than 6%. Interday and intraday variability were found to be less than 8.3%. The limit of quantitation and detection were 0.5 and 0.25 microgram ml-1 (s/n > 3), respectively, for both drug and metabolite.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
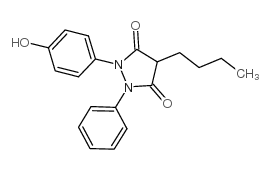 |
G-29701
CAS:129-20-4 |
C19H20N2O3 |
|
Bone marrow depression due to mianserin, phenylbutazone, oxy...
1986-07-01 [Adverse Drug React. Acute Poisoning Rev. 5(2) , 97-136, (1986)] |
|
Bone marrow depression due to mianserin, phenylbutazone, oxy...
1986-10-01 [Adverse Drug React. Acute Poisoning Rev. 5(3) , 181-96, (1986)] |
|
Disposition and tolerance of suxibuzone in horses.
1999-09-01 [Equine Vet. J. 31(5) , 411-6, (1999)] |
|
Pharmacokinetics of phenylbutazone and its metabolite oxyphe...
2001-05-01 [Am. J. Vet. Res. 62(5) , 673-5, (2001)] |
|
Screening, quantification, and confirmation of phenylbutazon...
2009-01-01 [J. Anal. Toxicol. 33(1) , 41-50, (2009)] |