Journal of Pharmacy and Pharmacology
1987-01-01
Solubilization kinetics of n-alkanes by a non-ionic surfactant.
A C Donegan, A J Ward
Index: J. Pharm. Pharmacol. 39(1) , 45-7, (1987)
Full Text: HTML
Abstract
The rates of solubilization of a homologous series of n-alkanes (CnH2n + 2; n = 8-16) into micellar solutions of n-dodecyl hexaoxyethylene glycol ether (C12E6) have been measured at 298 K. A mechanism involving a micellar dissociation-association process previously proposed was found to give an adequate description of the data. Proportionality between the micellar solubilization capacity in the kinetic process and that found at equilibrium could be inferred for such a series of solubilizates which have the same locus of solubilization and packing requirements.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
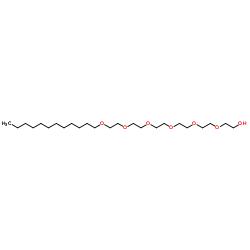 |
hexaethyleneglycol monododecyl ether
CAS:3055-96-7 |
C24H50O7 |
Related Articles:
More...
|
Size characterization of commercial micelles and microemulsi...
2015-08-15 [Int. J. Pharm. 492 , 46-54, (2015)] |
|
Analysis of the Photophysical Behavior and Rotational-Relaxa...
2015-01-01 [Molecules 20 , 19343-60, (2015)] |
|
Pressure-anesthetic antagonism on the phase separation of no...
1980-12-12 [Biochim. Biophys. Acta 603(2) , 237-44, (1980)] |
|
Absorption and elimination of a branched-chain alkylpolyetho...
1983-09-01 [Toxicol. Lett. 18(3) , 351-7, (1983)] |
|
Size change of the wormlike micelles of pentaoxyethylene, he...
2008-04-17 [J. Phys. Chem. B 112(15) , 4648-55, (2008)] |