Bioscience, Biotechnology, and Biochemistry
2004-06-01
Purification and characterization of a novel prolyl aminopeptidase from Maitake (Grifola frondosa).
Kazuyuki Hiwatashi, Kazuyuki Hori, Keitaro Takahashi, Akira Kagaya, Shunzo Inoue, Toshihiro Sugiyama, Saori Takahashi
Index: Biosci. Biotechnol. Biochem. 68 , 1395-7, (2004)
Full Text: HTML
Abstract
We have found a novel prolyl aminopeptidase in Grifola frondosa. The enzyme was purified by DEAE-Sepharose CL-6B, Butyl-Toyopearl, Sephacryl S-100, and Mono-Q column chromatographies. The purified enzyme exists as a dimer and gives high activity toward L-proline-p-nitroanilide. The enzyme was strongly inhibited by p-chloromercuribenzoic acid and iodoacetic acid and markedly inhibited by phenylmethylsulfonyl fluoride and arphamenin A.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
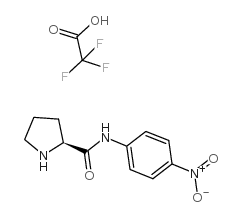 |
h-pro-pna tfa
CAS:108321-19-3 |
C13H14F3N3O5 |
Related Articles:
More...
|
Generation of food-grade recombinant Lactobacillus casei del...
2014-08-01 [Appl. Microbiol. Biotechnol. 98(15) , 6689-700, (2014)] |
|
Synthesis and biological evaluation of nigranoic acid esters...
2015-01-01 [Nat. Prod. Res. 29 , 1650-6, (2015)] |
|
Characterization of a multimeric, eukaryotic prolyl aminopep...
2009-11-01 [Microbiology 155 , 3673-3682, (2009)] |
|
Characterization of an aminopeptidase and a proline iminopep...
2008-01-01 [Z. Naturforsch., C, J. Biosci. 63 , 105-112, (2008)] |
|
Collagen production in cardiac fibroblasts during inhibition...
2005-09-01 [J. Renin Angiotensin Aldosterone Syst. 6 , 69-77, (2005)] |