| Structure | Name/CAS No. | Articles |
|---|---|---|
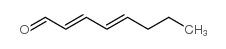 |
2,4-octadienal
CAS:5577-44-6 |
Giuliana d'Ippolito, Adele Cutignano, Sara Tucci, Giovanna Romano, Guido Cimino, Angelo Fontana
Index: Phytochemistry 67(3) , 314-22, (2006)
Full Text: HTML
Intermediates of the aldehyde biosynthesis in Thalassiosira rotula are investigated. Use of labeled precursors and cell preparations proves production of 2E,4Z-octadienal from 6Z,9Z,12Z-hexadecatrienoic acid (C16:3 omega-4) through the lipoxygenase-dependent intermediate (9S)-9-hydroperoxyhexadeca-6,10,12-trienoic acid. On the contrary, synthesis of 2E,4Z,7Z-decatrienal involves mainly EPA (C20:5 omega-3) by a 11R-lipoxygenase, as suggested by identification of chiral 11R-HEPE (12% e.e.) in the diatom extracts. Consistently with the necessity to have a rapid transport and metabolization of the intermediate hydroperoxides, we show that lipoxygenase and lyase activities are both found in the same subcellular fraction of the microalga.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
2,4-octadienal
CAS:5577-44-6 |
C8H12O |
|
The effect of dissolved polyunsaturated aldehydes on microzo...
2015-05-01 [Mar. Drugs 13 , 2834-56, (2015)] |
|
Dynamics of dissolved and particulate polyunsaturated aldehy...
2011-01-01 [Mar. Drugs 9 , 345-58, (2011)] |
|
Differential effect of three polyunsaturated aldehydes on ma...
2008-01-31 [Aquat. Toxicol. 86(2) , 249-55, (2008)] |
|
Production of octadienal in the marine diatom Skeletonema co...
2003-03-20 [Org. Lett. 5(6) , 885-7, (2003)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved