Steroids
1993-11-01
Synthesis of 7- and 12-hydroxy- and 7,12-dihydroxy-3-keto-5 beta-cholan-24-oic acids by reduction of 3,7-, 3,12- and 3,7,12-oxo derivatives.
G Fantin, M Fogagnolo, A Medici, P Pedrini, U Cova
Index: Steroids 58(11) , 524-6, (1993)
Full Text: HTML
Abstract
7 alpha-, 12 alpha-, 12 beta-Hydroxy and 7 alpha,12 alpha- and 7 alpha,12 beta-dihydroxy-3-ketocholanoic acids were prepared in satisfactory yields protecting the 3-keto group as dimethyl ketal and subsequent reduction with sodium borohydride of the corresponding 7- and 12-oxo functionalities. The same procedure gave also 3,12-diketo-7 alpha-hydroxy-cholanoic acid.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
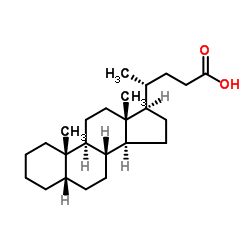 |
5β-cholanoic acid
CAS:546-18-9 |
C24H40O2 |
Related Articles:
More...
|
An efficient synthesis of 4 beta- and 6 alpha-hydroxylated b...
1993-02-01 [Steroids 58(2) , 52-8, (1993)] |
|
Calcium- and voltage-gated potassium (BK) channel activators...
2012-10-01 [ChemMedChem 7(10) , 1784-92, (2012)] |
|
Cholanic acids determined in commercial drugs by means of a ...
1993-01-01 [J. Pharm. Biomed. Anal. 11(11-12) , 1207-14, (1993)] |
|
Synthesis of [3,4-(13)c(2)]-enriched bile salts as NMR probe...
2002-09-20 [J. Org. Chem. 67(19) , 6764-71, (2002)] |
|
Microwave-induced organic reactions of bile acids: esterific...
1995-06-01 [Steroids 60(6) , 453-7, (1995)] |