Enzyme
1987-01-01
Determination and characterization of succinyl tri-alanine p-nitroanilide hydrolyzing metalloendopeptidase in serum.
M Ishida, M Ogawa, T Mori, T Mega
Index: Enzyme 37(4) , 202-7, (1987)
Full Text: HTML
Abstract
Serum succinyl (Ala)3-p-nitroanilide hydrolyzing elastase-like activity which elevates in patients with obstructive jaundice, is due to the joint action of two enzymes: first, succinyl (Ala)3-p-nitroanilide is cleaved to succinyl (Ala)2 and Ala-p-nitroanilide by metalloendopeptidase, and then Ala-p-nitroanilide is cleaved to Ala and p-nitroaniline by aminopeptidase. We adopt a new assay method for serum endopeptidase activity using HPLC.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
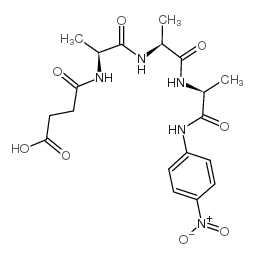 |
Suc-Ala-Ala-Ala-pNA
CAS:52299-14-6 |
C19H25N5O8 |
Related Articles:
More...
|
A new Kunitz-type plasmin inhibitor from scorpion venom.
2015-11-01 [Toxicon 106 , 7-13, (2015)] |
|
SjAPI, the first functionally characterized Ascaris-type pro...
2013-01-01 [PLoS ONE 8(3) , e57529, (2013)] |
|
Purification, kinetical and molecular characterizations of a...
1996-09-01 [Comp. Biochem. Physiol. B Biochem. Mol. Biol. 115(1) , 87-95, (1996)] |
|
Characterization of metalloelastase-like activity from the p...
1991-12-01 [Biol. Chem. Hoppe-Seyler 372 , 1057-1064, (1991)] |
|
Determination of elastase inhibitory activity of alpha 1-pro...
1987-06-01 [Scand. J. Clin. Lab. Invest. 47(4) , 405-10, (1987)] |