Journal of Organic Chemistry
2013-02-15
C-alkylation of chiral tropane- and homotropane-derived enamines.
David M Hodgson, Andrew Charlton, Robert S Paton, Amber L Thompson
Index: J. Org. Chem. 78(4) , 1508-18, (2013)
Full Text: HTML
Abstract
The synthesis and alkylation of chiral, nonracemic tropane- and homotropane-derived enamines is examined as an approach to enantioenriched α-alkylated aldehydes. The two bicyclic N auxiliaries, which differ by a single methylene group, give opposite senses of asymmetric induction on alkylation with EtI and provide modestly enantioenriched 2-ethylhexanal (following hydrolysis of the alkylated iminium). The observed stereoselectivity is supported by density functional studies of ethylation for both enamines.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
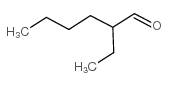 |
Hexanal, 2-ethyl
CAS:123-05-7 |
C8H16O |
Related Articles:
More...
|
Glutathionylation and Reduction of Methacrolein in Tomato Pl...
2015-11-01 [Plant Physiol. 169 , 1744-54, (2015)] |
|
Absorption and biodegradation of hydrophobic volatile organi...
2009-01-01 [Water Sci. Technol. 59(7) , 1315-22, (2009)] |
|
Effects of di-(2-ethylhexyl) phthalate and four of its metab...
2012-05-01 [Ecotoxicol. Environ. Saf. 79 , 108-15, (2012)] |
|
Metabolites from the biodegradation of di-ester plasticizers...
2006-07-31 [Sci. Total Environ. 366(1) , 286-94, (2006)] |
|
E-2-ethylhexenal, E-2-ethyl-2-hexenol, mellein, and 4-hydrox...
2008-02-01 [J. Chem. Ecol. 34(2) , 215-9, (2008)] |