Biotransformation of the diphenyl ether herbicide lactofen and purification of a lactofen esterase from Brevundimonas sp. LY-2.
Bo Liang, Yu-Kun Zhao, Peng Lu, Shun-Peng Li, Xing Huang
Index: J. Agric. Food Chem. 58(17) , 9711-5, (2010)
Full Text: HTML
Abstract
The diphenyl ether herbicide lactofen is commonly used to control broadleaf weeds. Once released into the environment, this herbicide is subject to microbial reactions. This study describes the biotransformation of lactofen by Brevundimonas sp. LY-2 isolated from enrichment cultures inoculated with soil sample. This strain degraded about 80% of 50 mg L(-1) lactofen in 5 days of incubation in flasks. The metabolic behaviors of the herbicide in the media are described. The results show a transformation pathway of lactofen by the bacterium leading to the formation of 1-(carboxy)ethyl-5-(2-chloro-4-(trifluoromethyl)phenoxy)-2-nitrobenzoate and ethanol. An esterase, which could cleave the right ester bond of the alkanoic side chain of lactofen, was purified 113.3-fold to homogeneity with 6.83% recovery. The current results suggested that Brevundimonas sp. LY-2 degraded lactofen via the ester bond cleavage catalyzed by esterase.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
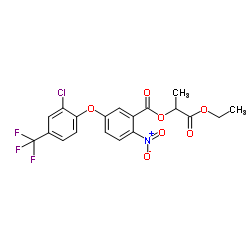 |
Lactofen
CAS:77501-63-4 |
C19H15ClF3NO7 |
|
Enantioselective degradation in sediment and aquatic toxicit...
2010-02-24 [J. Agric. Food Chem. 58(4) , 2439-45, (2010)] |
|
A codon deletion confers resistance to herbicides inhibiting...
2006-08-15 [Proc. Natl. Acad. Sci. U. S. A. 103(33) , 12329-34, (2006)] |
|
Influence of soil properties on the enantioselective dissipa...
2009-07-08 [J. Agric. Food Chem. 57(13) , 5865-71, (2009)] |
|
Induction of hepatic peroxisome proliferation in mice by lac...
1988-03-30 [Toxicol. Appl. Pharmacol. 93(1) , 72-80, (1988)] |
|
The diphenylether herbicide lactofen induces cell death and ...
2005-12-01 [Plant Physiol. 139(4) , 1784-94, (2005)] |