Synergism between trimethoprim and sulfonamide in urine: does it exist?
I D Watson, H N Cohen, S J McIntosh, J A Thomson, A Shenkin
Index: Clin. Ther. 4(2) , 103-8, (1981)
Full Text: HTML
Abstract
In a randomized, crossover, multiple dose study, ten healthy volunteers received the recommended dosages of cotrimoxazole (80 mg trimethoprim and 400 mg sulfamethoxazole) and co-tri-famole (80 mg trimethoprim and 400 mg sulfamoxole) for five days each. Urinary levels of trimethoprim and each sulfonamide were measured daily for five days. The urinary ratios of trimethoprim and sulfonamide for both formulations were consistently lower than those considered optimal for synergy. Concentration of trimethoprim in the urine from both preparations was found to be greatly in excess of the MIC for trimethoprim-sensitive urinary pathogens (approximately 2 microgram/ml). The sulfonamide levels achieved were not consistently in excess of their MIC (approximately 200 microgram/ml) for either preparation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
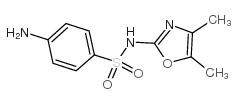 |
sulfamoxol
CAS:729-99-7 |
C11H13N3O3S |
|
Adverse reactions to sulphonamide and sulphonamide-trimethop...
1996-03-01 [Adverse Drug React. Toxicol. Rev. 15(1) , 9-50, (1996)] |
|
Combination of trimethoprim with sulfonamides other than sul...
1982-01-01 [Rev. Infect. Dis. 4(2) , 411-8, (1982)] |
|
Use of p-benzoquinone for the spectrophotometric determinati...
1991-01-01 [J. Pharm. Biomed. Anal. 9(7) , 531-8, (1991)] |
|
Comparative pharmacokinetics of co-trifamole and co-trimoxaz...
1982-09-01 [Br. J. Clin. Pharmacol. 14(3) , 437-43, (1982)] |
|
[Supristol suspension in pediatrics].
1980-12-01 [Lille Med. 25(10) , 611-2, (1980)] |