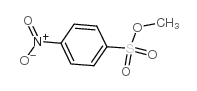
Study of the reaction between methyl 4-nitrobenzenesulfonate and bromide ions in mixed single-chain-gemini micellar solutions: kinetic evidence for morphological transitions.
María del Mar Graciani, Amalia Rodríguez, María Luisa Moyá
Index: J. Colloid. Interface Sci. 328(2) , 324-30, (2008)
Full Text: HTML
Abstract
The reaction between methyl 4-nitrobenzenesulfonate and bromide ions has been studied in mixed single-chain-gemini micellar solutions of n-dodecyltrimethylammonium bromide, DTAB, and dodecyl tricosaoxyethylene glycol ether, Brij(35), with alkanediyl-alpha-omega-bis(dodecyldimethylammonium) bromide, 12-s-12,2Br(-) (s=3,4,5). Kinetic micellar effects show that an increase in the solution mole fraction of the single-chain surfactant, X(single-chain), results in a diminution of the mixed micelles tendency to form spherocylindrical aggregates upon increasing surfactant concentration. The dependence of the surfactant concentration at which the sphere-to-rod transition occurs, C(*), on X(single-chain) showed through kinetic data was in agreement with results obtained by means of fluorescence measurements.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
(S)-3-(BOC-AMINO)-2-OXO-1-AZEPINE-ACETICACID
CAS:6214-20-6 |
C7H7NO5S |
|
Inhibition of inositol 1,3,4-trisphosphate 5/6-kinase by ami...
1993-11-01 [Biochem. Soc. Trans. 21(4) , 365S, (1993)] |
|
The chemical modification of Escherichia coli ribosomes with...
1980-12-01 [Can. J. Biochem. 58 , 1345, (1980)] |
|
Cysteine modification of metallothionein.
1991-01-01 [Meth. Enzymol. 205 , 399-400, (1991)] |
|
Uterotropic action in rats of amsonic acid and three of its ...
1992-05-01 [J. Toxicol. Environ. Health A 36(1) , 13-25, (1992)] |
|
Methylation of the active center histidine 217 in D-amino ac...
1984-05-10 [J. Biol. Chem. 259(9) , 5585-90, (1984)] |