| Structure | Name/CAS No. | Articles |
|---|---|---|
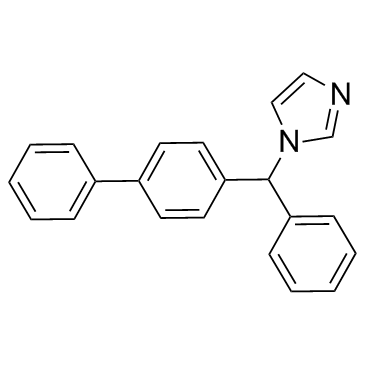 |
Bifonazole
CAS:60628-96-8 |
|
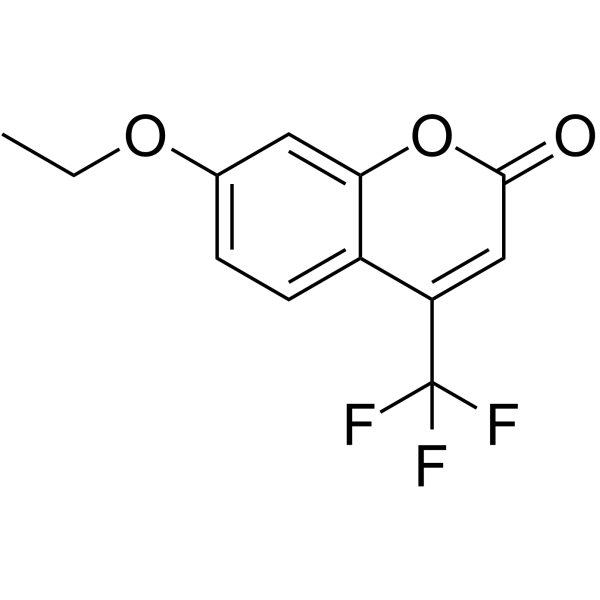 |
7-Ethoxy-4-(trifluoromethyl)coumarin
CAS:115453-82-2 |