Journal of the American Chemical Society
2008-11-12
Asymmetric synthesis of bicyclo[4.3.1]decadienes and bicyclo[3.3.2]decadienes via [6 + 3] trimethylenemethane cycloaddition with tropones.
Barry M Trost, Patrick J McDougall, Olaf Hartmann, Peter T Wathen
Index: J. Am. Chem. Soc. 130(45) , 14960-1, (2008)
Full Text: HTML
Abstract
The cyanosubstituted trimethylenemethane donor undergoes palladium-catalyzed [6 + 3] cycloaddition with a variety of tropones to yield bicyclo[4.3.1]decadienes in excellent regio-, diastereo-, and enantioselectivity. Products of the Pd-TMM [6 + 3] cycloaddition participate in a thermal [3,3] sigmatropic rearrangement to yield bicyclo[3.3.2]decadienes in good yield.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
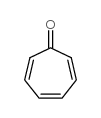 |
TROPONE
CAS:539-80-0 |
C7H6O |
Related Articles:
More...
|
Benzo[cd]azulene skeleton: azulene, heptafulvene, and tropon...
2009-12-03 [Org. Lett. 11(23) , 5363-5, (2009)] |
|
Tropylium tetrafluoroborate, a novel substrate for aldehyde ...
1986-10-30 [Biochem. Biophys. Res. Commun. 140(2) , 609-15, (1986)] |
|
Potential of Piperazinylalkylester Prodrugs of 6-Methoxy-2-N...
2015-06-01 [Z. Naturforsch., C, J. Biosci. 59(3-4) , 184-6, (2004)] |
|
Pernambucone, a new tropone derivative from Croton argyroglo...
2009-05-01 [Pharmazie 64(5) , 350-1, (2009)] |
|
Metal-catalyzed [6 + 3] cycloaddition of tropone with azomet...
2014-02-12 [J. Am. Chem. Soc. 136(6) , 2625-9, (2014)] |