The pH dependency of bovine spleen cathepsin B-catalyzed transfer of N alpha-benzyloxycarbonyl-L-lysine from p-nitrophenol to water and dipeptide nucleophiles. Comparisons with papain.
A S Bajkowski, A Frankfater
Index: J. Biol. Chem. 258(3) , 1650-5, (1983)
Full Text: HTML
Abstract
Cathepsin B has been shown to catalyze the transfer of the N alpha-benzyloxycarbonyl-L-lysyl residue from the corresponding p-nitrophenyl ester substrate to water and dipeptide nucleophiles. These reactions occurred through the formation of an acyl-enzyme intermediate. The pH dependency of the acylation and deacylation steps were determined from the increases in the maximum rate of appearance of p-nitrophenol on addition of glycylglycine or L-leucylglycine to the reaction. The second order acylation rate constant, kcat/Km was found to depend on the state of ionization of three groups in the enzyme having pKa values of 4.2, 5.5, and 8.6. Protonation of the group with pKa = 5.5 decreased but did not abolish enzymatic activity, resulting in the appearance of a second, active protonic form of the enzyme between pH 4.2 and pH 5.5. The first order rate constant for the hydrolysis of the acyl-enzyme intermediate was independent of pH between 4.0 and 7.5. In contrast, acyl group transfer from cathepsin B to glycylglycine and L-leucylglycine depended on a group with a pKa of about 4.5. These results are discussed in terms of possible structural and functional homologies between the active sites of cathepsin B and papain.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
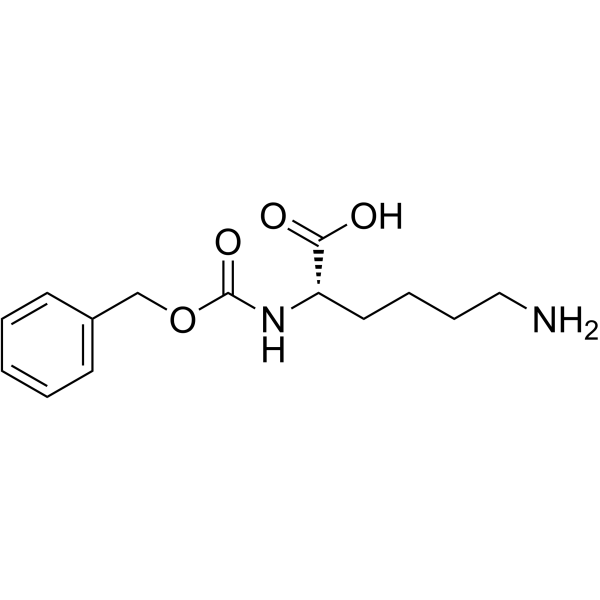 |
Z-Lys-OH
CAS:2212-75-1 |
C14H20N2O4 |
|
Nanofibrous spongy microspheres enhance odontogenic differen...
2015-09-16 [Adv. Healthc. Mater. 4 , 1993-2000, (2015)] |
|
Characterization of Nalpha-benzyloxycarbonyl-L-lysine oxidiz...
2007-09-01 [J. Biosci. Bioeng. 104(3) , 218-23, (2007)] |
|
Enzymes responsible for the conversion of N alpha-[(Benzylox...
2010-06-01 [Chem. Biodivers. 7(6) , 1549-54, (2010)] |