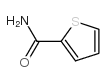
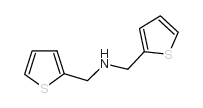
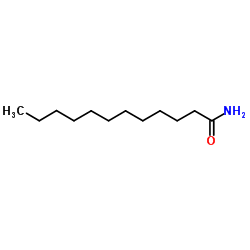
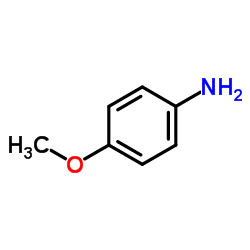
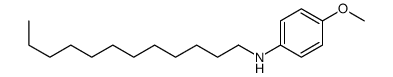
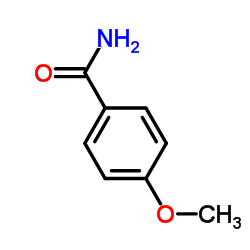
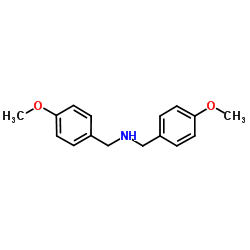
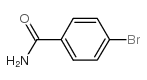
|
~84% |
|
~54% |
|
~0% |
|
~71% |
|
~40% |
|
~89% |