Geiparvarin analogues. 4. Synthesis and cytostatic activity of geiparvarin analogues bearing a carbamate moiety or a furocoumarin fragment on the alkenyl side chain.
S Manfredini, P G Baraldi, R Bazzanini, M Guarneri, D Simoni, J Balzarini, E De Clercq
Index: J. Med. Chem. 37 , 2401, (1994)
Full Text: HTML
Abstract
As a continuation of previous studies on the synthesis and antitumor activity of geiparvarin analogues bearing a carbamate moiety in the alkyl side chain, a series of N-substituted [(E)-3-(4,5-dihydro-5,5-dimethyl-4-oxo-2-furanyl)-2- butenyl]carbamates (15a-f) were synthesized and tested with the objective to investigate the reason for the marked difference of cytostatic activity found between alkyl and phenyl derivatives. A series of compounds, characterized by different physicochemical properties, were designed in order to study this hypothesis. Moreover, to further investigate the modification of the alkenyl side chain, (E)- and (Z)-[2-(4,5-dihydro-5,5-dimethyl-4-oxo-2-furanyl)propenyl]-7H-furo[3,2- g][1]benzopyran-7-one (11a,b) were synthesized, the latter compounds being the combination of two units, namely, the 3(2H)-furanone ring system endowed with potent alkylating properties and the furocoumarin portion which binds to DNA resulting in potential DNA-targeted alkylating agents. The compounds were tested for their cytostatic activity against proliferation of murine (L1210) and human (Molt/4, CEM, or MT-4) tumor cells. The highest cytostatic activity found within both series of carbamic derivatives (15a-d,k and 15e,g-j) was associated with the highest global lipophilicity. With regard to compounds 11a,b, the cytostatic activity of (Z)-furocoumarin 11b might be related to a specific interaction with DNA (i.e., intercalation).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
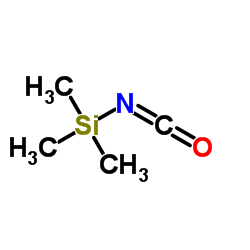 |
trimethylsilyl isocyanate
CAS:1118-02-1 |
C4H9NOSi |
|
Covalent modification of a metal-organic framework with isoc...
2008-08-07 [Chem. Commun. (Camb.) (29) , 3366-8, (2008)] |
|
[J. Prakt. Chem./Chem.-Ztg. 335 , 558, (1993)] |
|
[Tetrahedron Lett. 48 , 6002, (2007)] |
|
Reactions of trimethylsilyl isocyanate and isothiocyanate wi...
[J. Org. Chem. 53(22) , 5298-5300, (1988)] |
|
SiNCO+ and SiNCS+ and their neutral co...
[Chem. Phys. Lett. 316(3) , 243-7, (2000)] |