Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma.
Paul A Meyers
Index: Expert Rev. Anticancer Ther. 9(8) , 1035-49, (2009)
Full Text: HTML
Abstract
Osteosarcoma is an ultraorphan disease. There are approximately 1000 new patients diagnosed with osteosarcoma each year in the USA and Europe. Current treatment for osteosarcoma utilizes multiagent chemotherapy and surgical resection of all clinically detectable disease. Current treatments for osteosarcoma achieve 60-70% event-free survival (EFS) for patients with localized disease and approximately 20% EFS for patients who present with metastasis. These results have been stable for two decades. The addition of muramyl tripeptide (mifamurtide) to chemotherapy resulted in a trend towards improved EFS and a one-third reduction in the risk of death from osteosarcoma. Mifamurtide has been approved in Europe for the treatment of newly diagnosed osteosarcoma in combination with chemotherapy.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
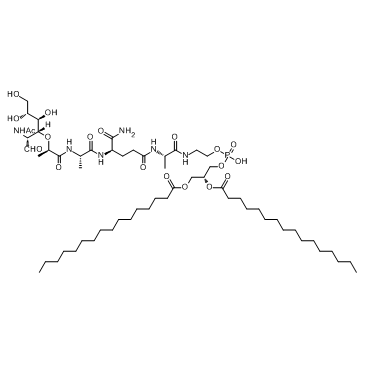 |
Mifamurtide
CAS:83461-56-7 |
C59H109N6O19P |
|
Imunomodulative effect of liposomized muramyltripeptide phos...
2008-09-01 [Parasitol. Res. 103(4) , 919-29, (2008)] |
|
In vitro and in vivo enhancement of canine pulmonary alveola...
1999-04-01 [Cancer Biother. Radiopharm. 14(2) , 121-8, (1999)] |
|
Liposomal muramyl tripeptide (CGP 19835A lipid) therapy for ...
1998-10-01 [Cancer Biother. Radiopharm. 13(5) , 363-8, (1998)] |
|
Effect of MTP on TNF-alpha in perfused rat liver after bacte...
1998-05-01 [J. Surg. Res. 76(2) , 179-84, (1998)] |
|
Liposomal muramyl tripeptide phosphatidyl ethanolamine: a sa...
2008-02-01 [Expert Rev. Anticancer Ther. 8(2) , 151-9, (2008)] |