Lead detoxification activities and ADMET hepatotoxicities of a class of novel 5-(1-carbonyl-L-amino-acid)-2,2-dimethyl-[1,3]dithiolane-4-carboxylic acids.
Yanxia Xu, Yuji Wang, Ming Zhao, Baoguang Hou, Li Peng, Meiqing Zheng, Jianhui Wu, Shiqi Peng
Index: Bioorg. Med. Chem. Lett. 21 , 1754-7, (2011)
Full Text: HTML
Abstract
By linking the mercapto groups with isopropyl and introducing L-amino acid into the 5-carboxyl of DMSA a class of novel 5-(1-carbonyl-L-amino-acid)-2,2- dimethyl-[1,3]dithiolane-4-carboxylic acids were prepared. Their in vivo activities were evaluated on lead loaded mice at the dose of 0.4 mmol/kg. The results showed that the lead levels of the livers, kidneys, femurs and brains in particular could be efficiently decreased by 0.4 mmol/kg of 5-(1-carbonyl-L-amino-acid)-2,2-dimethyl-[1,3]dithiolane-4-carboxylic acids. The benefit of 5-(1-carbonyl-L-amino-acid)-2,2-dimethyl-[1,3]dithiolane-4-carboxylic acids to the detoxification of the brain lead was attributed to their transmembrane ability. Compared with the lead detoxification efficacy, they did not affect the essential metals such as Fe, Cu, Zn, and Ca of the treated mice. Silico molecular modeling predicted that 5-(1-carbonyl-L-amino-acid)-2,2-dimethyl-[1,3]dithiolane-4-carboxylic acids had no hepatotoxicity.Crown Copyright © 2011. Published by Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
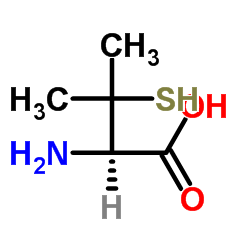 |
L-(+)-Penicillamine
CAS:52-66-4 |
C5H11NO2S |
|
Identification and Characterization of EDTA Test Strip Inter...
2015-01-01 [Clin. Lab. 61 , 785-91, (2015)] |
|
Paternal breed effects on expression of IGF-II, BAK1 and BCL...
2015-10-01 [Zygote 23 , 712-21, (2015)] |
|
All-trans retinoic acid prevents oxidative stress-induced lo...
2015-05-01 [J. Nutr. Biochem. 26 , 441-54, (2015)] |
|
N-Terminal 2,3-diaminopropionic acid (Dap) peptides as effic...
2009-01-01 [Bioorg. Med. Chem. 17 , 2310-20, (2009)] |
|
Interferon-gamma and nitric oxide synthase 2 mediate the agg...
2015-01-01 [PLoS ONE 10 , e0128301, (2015)] |