Regioselective dioxygenation of ortho-trifluoromethylbenzoate by Pseudomonas aeruginosa 142: evidence for 1,2-dioxygenation as a mechanism in ortho-halobenzoate dehalogenation.
S A Selifonov, J E Gurst, L P Wackett
Index: Biochem. Biophys. Res. Commun. 213(3) , 759-67, (1995)
Full Text: HTML
Abstract
Pseudomonas aeruginosa strain 142 oxidizes 2-halobenzoates via a multicomponent oxygenase (V. Romanov and R.P. Hausinger, J. Bacteriol., 1994, 176(11), 3368-3374). The intermediacy of a highly unstable cis-diol in the reaction has been proposed. Direct evidence for this is currently lacking and the stereochemical course of the reaction cannot be inferred from previous studies. In this study, 2-trifluoromethylbenzoate was stoichiometrically oxidized by P. aeruginosa 142 to a chiral product identified as (-)2-trifluoromethyl-cis-1,2-dihydroxy-3,5-cyclohexadiene-1-carboxylic acid. These data rigorously establish a dioxygenative mechanism for 2-halobenzoate metabolism.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
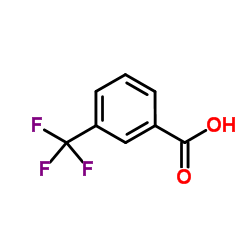 |
3-(Trifluoromethyl)benzoic acid
CAS:454-92-2 |
C8H5F3O2 |
|
The many roles for fluorine in medicinal chemistry.
2008-08-14 [J. Med. Chem. 51 , 4359-69, (2008)] |
|
Studies on the metabolism of fluorinated xenobiotics in the ...
1990-01-01 [J. Pharm. Biomed. Anal. 8(8-12) , 939-44, (1990)] |
|
Bacterial metabolism of side chain fluorinated aromatics: co...
1988-01-01 [Arch. Microbiol. 149(3) , 188-97, (1988)] |
|
Degradation of meta-trifluoromethylbenzoate by sequential mi...
1993-06-15 [FEMS Microbiol. Lett. 110(2) , 213-6, (1993)] |
|
Polymeric drugs with prolonged sustained delivery of specifi...
2004-01-01 [J. Biomater. Sci. Polym. Ed. 15(7) , 917-28, (2004)] |