Effect of human C-reactive protein on chemokine and chemotactic factor-induced neutrophil chemotaxis and signaling.
W Zhong, Q Zen, J Tebo, K Schlottmann, M Coggeshall, R F Mortensen
Index: J. Immunol. 161(5) , 2533-40, (1998)
Full Text: HTML
Abstract
C-reactive protein (CRP) is a unique serum pentraxin and the prototype acute phase reactant. CRP is a ligand for specific receptors on phagocytic leukocytes, and mediates activation reactions of monocytes/macrophages, but inhibits the respiratory burst of neutrophils (PMN). Since CRP selectively accumulates at inflammatory sites in which IL-8 is also produced, we tested the effects of CRP on the responsiveness of PMN to IL-8 and the bacterial chemotactic peptide, FMLP-phenylalanine (FMLPP). Purified human CRP inhibited the chemotactic response of PMN to IL-8 and FMLPP. A mouse IgM mAb that was generated against the leukocyte CRP receptor (CRP-R) also inhibited the chemotactic response. Incubation of purified CRP with activated PMN generated CRP-derived peptides that also inhibited chemotaxis. A synthetic CRP peptide (residues 27-38) that binds to the CRP-R had weak chemotactic activity, whereas two other CRP synthetic peptides (residues 174-185 and 191-205) inhibited chemotaxis of PMNs to both IL-8 and FMLPP. CRP did not alter receptor-specific binding of IL-8, but exerted its effect at the level of signaling. CRP augmented both IL-8- and FMLPP-induced mitogen-activated protein kinase (extracellular signal-regulated kinase-2) activity. CRP at acute phase levels increased both agonist-induced and noninduced phosphatidylinositol-3 kinase activity. The results suggest a role for CRP as a regulator of leukocyte infiltration at inflammatory sites.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
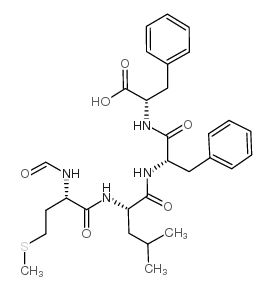 |
For-Met-Leu-Phe-Phe-OH
CAS:80180-63-8 |
C30H40N4O6S |
|
Formyl peptide chemoattractants: a model of the receptor on ...
1982-01-19 [Biochemistry 21 , 257, (1982)] |
|
Control of fluid shear response in circulating leukocytes by...
2002-03-01 [Ann. Biomed. Eng. 30(3) , 333-43, (2002)] |
|
Characteristics of binding of a potent chemotactic formyl te...
1988-05-01 [J. Leukoc. Biol. 43(5) , 420-8, (1988)] |
|
Interaction of Bordetella pertussis virulence components wit...
1988-08-01 [J. Gen. Microbiol. 134(8) , 2201-11, (1988)] |
|
A novel fluorescent cross-reactive formylpeptide receptor/fo...
2009-03-01 [Cytometry. A 75(3) , 264-70, (2009)] |