| Structure | Name/CAS No. | Articles |
|---|---|---|
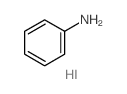 |
Benzenamine, hydriodide(1:1)
CAS:45497-73-2 |
|
 |
methylazanium iodide
CAS:14965-49-2 |
|
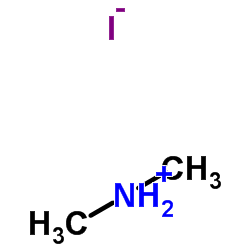 |
N-Methylmethanaminium iodide
CAS:51066-74-1 |
|
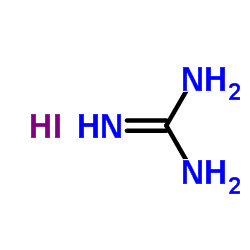 |
Guanidine Hydroiodide
CAS:19227-70-4 |
|
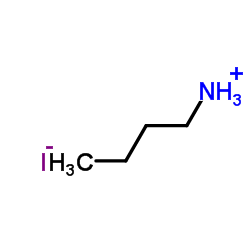 |
Butylamine Hydroiodide
CAS:36945-08-1 |
|
 |
Imidazole Hydroiodide
CAS:68007-08-9 |
|
 |
Propylamine Hydroiodide
CAS:14488-45-0 |
|
 |
Diethylamine Hydroiodide
CAS:19833-78-4 |
|
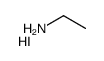 |
Ethylamine Hydroiodide
CAS:506-58-1 |
|
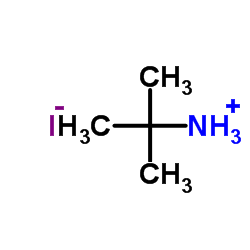 |
tert-Butylamine Hydroiodide
CAS:39557-45-4 |