Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid.
M Tanahashi, T Matsuda
Index: J. Biomed. Mater. Res. 34 , 305, (1997)
Full Text: HTML
Abstract
Self-assembled monolayers (SAMs) of alkanethiols having CH3, PO4H2, COOH, CONH2, OH, and NH2 terminal groups formed on a gold surface via sulfur attachment were soaked in a simulated body fluid (SBF), whose ion concentrations were nearly equal to those of human blood plasma, at 37 degrees C for up to 40 days. The effect of their terminal functional groups on apatite formation was assessed using X-ray photo-electron spectroscopic (XPS) measurement and a quartz crystal microbalance (QCM) technique. The Ca and P atoms were detected, of which element intensities increased with time, on SAMs except for the alkanethiol having the methyl terminal group. The Ca/P atomic ratios of the apatites formed on the SAMs ranged from around 1.0 to around 1.3. The most potent inducer for apatite formation, judged from the growth rate (micrometers per day) calculated from the weight change during QCM measurement, was the SAM of the alkanethiol with the PO4H2 group, followed by that of the alkanethiol with the COOH group. The SAMs of the alkanethiols with the CONH2, OH, and NH2 groups possessed much weaker inducing powers than the former two SAMs. Little weight change was observed for the methyl-group-terminated alkanethiol SAM. The growth rates increased with time, irrespective of the terminal group species among apatite formation-inducing groups. During the experimental observation period, the following relationship held. The growth rate decreased in the order PO4H2 > COOH >> CONH2 approximately equal to OH > NH2 >> CH3 approximately equal to 0. Some negatively charged groups strongly induced apatite formation but the positively charged group did not, it can be said that the apatite formation initiated via calcium ion-absorption upon complexation with a negative surface-charged group may be dominant in biomaterial calcification where ionic species directly contact the biomaterial surface in body fluids.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
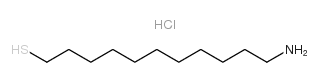 |
11-Amino-1-undecanethiol hydrochloride
CAS:143339-58-6 |
C11H26ClNS |
|
Genetically-controlled Vesicle-Associated Membrane Protein 1...
2015-01-01 [Molecular Neurodegeneration 10 , 18, (2015)] |
|
Temporin-SHa peptides grafted on gold surfaces display antib...
2014-07-01 [J. Pept. Sci. 20(7) , 563-9, (2014)] |
|
SERR Spectroelectrochemical Study of Cytochrome cd1 Nitrite ...
2015-01-01 [PLoS ONE 10 , e0129940, (2015)] |
|
Effect of surface chemistry on the integrin induced pathway ...
2015-02-01 [Colloids Surf. B Biointerfaces 126 , 188-97, (2015)] |
|
Evaluation of Chemical Interactions between Small Molecules ...
2015-01-01 [Sensors (Basel.) 15 , 30683-92, (2015)] |