| Structure | Name/CAS No. | Articles |
|---|---|---|
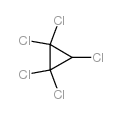 |
pentachlorocyclopropane
CAS:6262-51-7 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
pentachlorocyclopropane
CAS:6262-51-7 |