Spectroscopic studies on interaction and sonodynamic damage of metallochlorophyllin (Chl-M (M=Fe, Zn and Cu)) to protein under ultrasonic irradiation.
Jingqun Gao, Zhiqiu Wang, Jun Wang, Xudong Jin, Yuwei Guo, Kai Li, Ying Li, Pingli Kang
Index: Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 79(5) , 849-57, (2011)
Full Text: HTML
Abstract
In this paper, the chlorophyll derivatives, metallochlorophyllin (Chl-M) (M=Fe, Zn and Cu) including chlorophyllin iron (Chl-Fe), chlorophyllin zinc (Chl-Zn) and chlorophyllin copper (Chl-Cu), were adopted as sonosensitizers to combine with ultrasonic irradiation, and the sonodynamic damage of bovine serum albumin (BSA) was investigated. At first, the interaction of Chl-M with BSA was studied by fluorescence spectroscopy. The results show that the quenching mechanism belongs to a static process and among them the affinity of Chl-Fe to BSA is the most obvious. Then, some influence factors on the sonodynamic damage of BSA molecules in the presence of Chl-M under ultrasonic irradiation were also studied. Synchronous fluorescence spectra show that the binding and damage sites of Chl-M to BSA molecule are mainly on the tryptophan (Trp) residues. The generation of ROS in Chl-M sonodynamic process is estimated by the method of Oxidation-Extraction Spectrometry (OEP). This paper may offer some valuable references for the study of the sonodynamic activity of Chl-M and the effect of the central metals. Synchronously, it contributes to the application of Chl-M in SDT for tumor treatment.Copyright © 2011 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
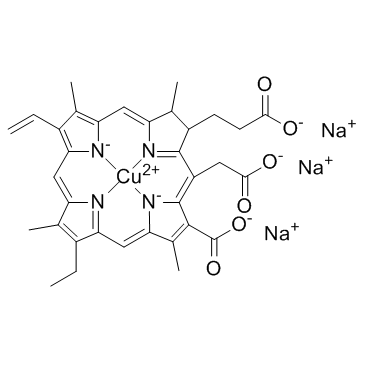 |
Chlorophyllin
CAS:11006-34-1 |
C34H31CuN4Na3O6 |
|
Phototocatalytic lithography of poly(propylene sulfide) bloc...
2009-01-20 [Langmuir 25 , 1238-44, (2009)] |
|
Assessment of the protective effects of selected dietary ant...
2011-08-01 [Food Chem. Toxicol. 49(8) , 1674-83, (2011)] |
|
Comparative proteomic analysis of drug sodium iron chlorophy...
2012-09-21 [Analyst 137(18) , 4287-94, (2012)] |
|
Chlorophyll derivative mediated PDT versus methotrexate: an ...
2012-12-01 [Photodiagnosis Photodyn. Ther. 9(4) , 362-8, (2012)] |
|
Oxidation-extraction spectrometry of reactive oxygen species...
2011-09-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 79(5) , 1099-104, (2011)] |