Biophysical analyses of designed and selected mutants of protocatechuate 3,4-dioxygenase1.
C Kent Brown, Matthew W Vetting, Cathleen A Earhart, Douglas H Ohlendorf
Index: Annu. Rev. Microbiol. 58 , 555-85, (2004)
Full Text: HTML
Abstract
The catechol dioxygenases allow a wide variety of bacteria to use aromatic compounds as carbon sources by catalyzing the key ring-opening step. These enzymes use specifically either catechol or protocatechuate (2,3-dihydroxybenozate) as their substrates; they use a bare metal ion as the sole cofactor. To learn how this family of metalloenzymes functions, a structural analysis of designed and selected mutants of these enzymes has been undertaken. Here we review the results of this analysis on the nonheme ferric iron intradiol dioxygenase protocatechuate 3,4-dioxygenase.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
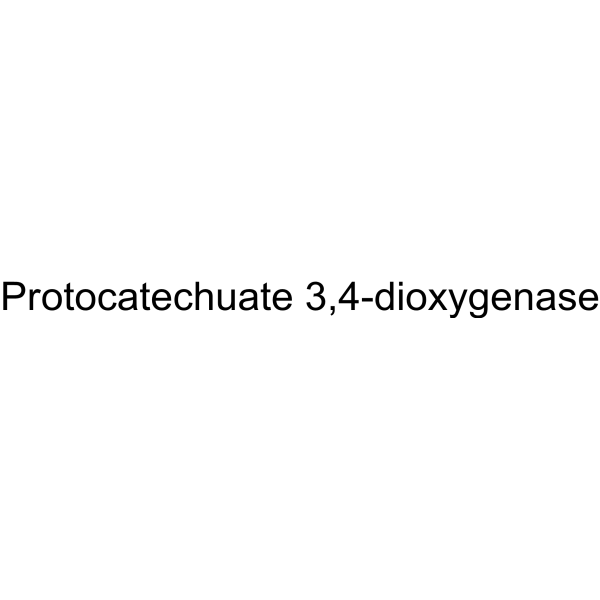 |
Protocatechuate 3,4-dioxygenase
CAS:9029-47-4 |
|
Motor domain phosphorylation modulates kinesin-1 transport.
2013-11-08 [J. Biol. Chem. 288(45) , 32612-21, (2013)] |
|
Role of Acinetobacter baylyi Crc in catabolite repression of...
2009-04-01 [J. Bacteriol. 191(8) , 2834-42, (2009)] |
|
Protocatechuic acid oxidase.
1954-10-01 [J. Biol. Chem. 210(2) , 799-808, (1954)] |
|
pcaH, a molecular marker for estimating the diversity of the...
2007-05-01 [Pest Manag. Sci. 63(5) , 459-67, (2007)] |
|
Spectroscopic studies of the effect of ligand donor strength...
2003-01-27 [Inorg. Chem. 42(2) , 365-76, (2003)] |