| Structure | Name/CAS No. | Articles |
|---|---|---|
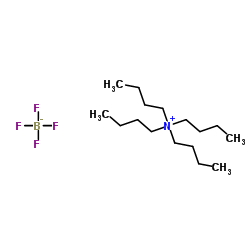 |
Tetrabutylammonium tetrafluoroborate
CAS:429-42-5 |
Andrew P Abbott, Eric G Hope, Donna J Palmer
Index: Anal. Chem. 77(20) , 6702-8, (2005)
Full Text: HTML
The viscosity of a supercritical electrolyte solution is measured for the first time using a modified quartz crystal microbalance, and it is shown that ionic solvation leads to a significant structuring of the solvent and an appreciable increase in solution viscosity. Voltammetric investigations in the electrolyte solutions are used to confirm the magnitude of the viscosity changes, and these account for the appreciably lower than expected peak currents.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Tetrabutylammonium tetrafluoroborate
CAS:429-42-5 |
C16H36BF4N |
|
Diamond nanowires modified with poly[3-(pyrrolyl)carboxylic ...
2014-09-07 [Analyst 139(17) , 4343-9, (2014)] |
|
Synthesis and spectral characterization of novel compounds d...
2003-05-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 59(7) , 1655-62, (2003)] |
|
A fluoride-releasing composite for dental applications.
2001-03-01 [Dent. Mater. 17(2) , 127-33, (2001)] |
|
Dentin pretreatment and caries inhibition by a fluoride-rele...
1993-08-01 [Am. J. Dent. 6(4) , 204-6, (1993)] |
|
Electrodeposition of metals from supercritical fluids.
2009-09-01 [Proc. Natl. Acad. Sci. U. S. A. 106(35) , 14768-72, (2009)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved