Zn(OTf)2-catalyzed reactions of ethenetricarboxylates with 2-aminobenzaldehydes leading to tetrahydroquinoline derivatives.
Shoko Yamazaki, Masachika Takebayashi, Kazuya Miyazaki
Index: J. Org. Chem. 75(4) , 1188-96, (2010)
Full Text: HTML
Abstract
Quinolines are an important class of compounds, and the development of new efficient synthetic strategies for the construction of quinolines is of considerable interest. Zinc triflate catalyzed cyclization of ethenetricarboxylate derivatives with 2-aminobenzaldehydes has been examined. The reaction of ethenetricarboxylate with 2-aminobenzaldehydes in the presence of zinc triflate (0.2 equiv) at 80 degrees C in ClCH(2)CH(2)Cl gave bridged tetrahydroquinoline derivatives in 15-95% yield. On the other hand, the reaction at room temperature in CH(2)Cl(2) gave hydroxy tetrahydroquinoline derivatives in 38-90% yield. Heating the hydroxy tetrahydroquinolines with zinc triflate (0.2 equiv) at 80 degrees C in ClCH(2)CH(2)Cl led to the bridged tetrahydroquinoline derivatives in 75-96% yield. Thermal reaction of the bridged tetrahydroquinolines (180 degrees C) gave indole derivatives regioselectively.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
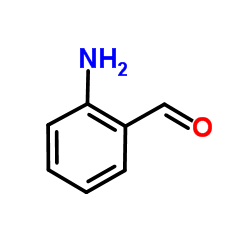 |
Formylaniline
CAS:529-23-7 |
C7H7NO |
|
Chondrogenesis of human bone marrow mesenchymal stromal cell...
2015-05-01 [Mater. Sci. Eng. C. Mater. Biol. Appl. 50 , 160-72, (2015)] |
|
B-ring-aryl substituted luotonin A analogues with a new bind...
2014-01-01 [PLoS ONE 9(5) , e95998, (2014)] |
|
The consequences of the isotope effect on proline dehydrogen...
1992-06-01 [Anal. Biochem. 203(2) , 191-200, (1992)] |
|
Co-regulation of mitochondrial respiration by proline dehydr...
2016-03-01 [Amino Acids 48 , 859-72, (2016)] |
|
Functional specialization in proline biosynthesis of melanom...
2012-01-01 [PLoS ONE 7 , e45190, (2012)] |