Synthesis and structure-activity relationships of o-sulfonamido-arylhydrazides as inhibitors of LL-diaminopimelate aminotransferase (LL-DAP-AT).
Chenguang Fan, John C Vederas
Index: Org. Biomol. Chem. 10(30) , 5815-9, (2012)
Full Text: HTML
Abstract
Recently, LL-diaminopimelate aminotransferase (LL-DAP-AT), a pyridoxal-5'-phosphate (PLP)-dependent enzyme, was reported to catalyze a key step in the biosynthesis of L-lysine in plants and Chlamydia. Previous screening of a 29,201-compound library against LL-DAP-AT identified an o-sulfonamidoarylhydrazide as a reversible inhibitor with IC(50)∼ 5 μM. Structure-activity relationship (SAR) studies based on this lead compound identified key structural features essential for enzyme inhibition and led to slightly improved inhibitors. Preliminary studies on the mode of inhibition of LL-DAP-AT by this class of compounds are also reported.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
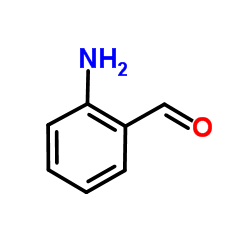 |
Formylaniline
CAS:529-23-7 |
C7H7NO |
|
Chondrogenesis of human bone marrow mesenchymal stromal cell...
2015-05-01 [Mater. Sci. Eng. C. Mater. Biol. Appl. 50 , 160-72, (2015)] |
|
B-ring-aryl substituted luotonin A analogues with a new bind...
2014-01-01 [PLoS ONE 9(5) , e95998, (2014)] |
|
The consequences of the isotope effect on proline dehydrogen...
1992-06-01 [Anal. Biochem. 203(2) , 191-200, (1992)] |
|
Co-regulation of mitochondrial respiration by proline dehydr...
2016-03-01 [Amino Acids 48 , 859-72, (2016)] |
|
Functional specialization in proline biosynthesis of melanom...
2012-01-01 [PLoS ONE 7 , e45190, (2012)] |