Phosphate ester hydrolysis by hydroxo complexes of trivalent lanthanides stabilized by 4-imidazolecarboxylate.
Francisco Aguilar-Pérez, Paola Gómez-Tagle, Elisa Collado-Fregoso, Anatoly K Yatsimirsky
Index: Inorg. Chem. 45(23) , 9502-17, (2006)
Full Text: HTML
Abstract
The anion of 4-imidazolecarboxylic acid (HL) stabilizes hydroxo complexes of trivalent lanthanides of the type ML(OH)+ (M = La, Pr) and M2L(n)(OH)(6-n) (M = La, n = 2; M = Pr, n = 2, 3; M = Nd, Eu, Dy, n = 1-3). Compositions and stability constants of the complexes have been determined by potentiometric titrations. Spectrophotometric and (1)H NMR titrations with Nd(III) support the reaction model for the formation of hydroxo complexes proposed on the basis of potentiometric results. Kinetics of the hydrolysis of two phosphate diesters, bis(4-nitrophenyl) phosphate (BNPP) and 2-hydroxypropyl 4-nitrophenyl phosphate (HPNPP), and a triester, 4-nitrophenyl diphenyl phosphate (NPDPP), in the presence of hydroxo complexes of five lanthanides were studied as a function of pH and metal and ligand concentrations. With all lanthanides and all substrates, complexes with the smallest n, that is M2L2(OH)4 for La and Pr and M2L(OH)5 for Nd, Eu, and Dy, exhibited the highest catalytic activity. Strong inhibitory effects by simple anions (Cl-, NO3-, (EtO)2PO2-, AcO-) were observed indicating high affinity of neutral hydroxo complexes toward anionic species. The catalytic activity decreased in the order La > Pr > Nd > Eu > Dy for both diester substrates and was practically independent of the nature of cation for a triester substrate. The efficiency of catalysis, expressed as the ratio of the second-order rate constant for the ester cleavage by the hydroxo complex to the second-order rate constant for the alkaline hydrolysis of the respective substrate, varied from ca. 1 for NPDPP to 10(2) for HPNPP and to 10(5) for BNPP. The proposed mechanism of catalytic hydrolysis involves reversible bridging complexation of a phosphodiester to the binuclear active species followed by attack on the phosphoryl group by bridging hydroxide (BNPP) or by the alkoxide group of the deprotonated substrate (HPNPP).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
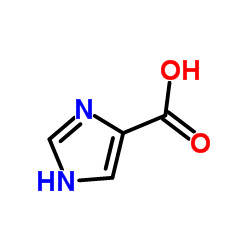 |
1H-Imidazole-4-carboxylic acid
CAS:1072-84-0 |
C4H4N2O2 |
|
AICAR administration affects glucose metabolism by upregulat...
2015-09-01 [Vet. J. 205 , 381-6, (2015)] |
|
Hair analysis of histamine and several metabolites in C3H/He...
2007-03-01 [Clin. Chim. Acta 378(1-2) , 122-7, (2007)] |
|
[Sci. Synth. 12 , 325, (2002)] |
|
catena-Poly [copper (I)-bis [μ-3-(1H-imidazol-2-...
[Acta Crystallogr. Sect. E Struct. Rep. Online 67(9) , 1288, (2011)] |
|
Synthesis and catalytic properties of imidazole-functionaliz...
[Bull. Korean Chem. Soc. 23(5) , 647-654, (2002)] |