Effects of atomoxetine on locomotor activity and impulsivity in the spontaneously hypertensive rat.
Michael Turner, Emma Wilding, Emmet Cassidy, Eleanor J Dommett
Index: Behav. Brain Res. 243 , 28-37, (2013)
Full Text: HTML
Abstract
Atomoxetine (ATX) is a commonly used non-stimulant treatment for Attention deficit hyperactivity disorder (ADHD). It primarily acts to increase noradrenalin levels; however, at higher doses it can increase dopamine levels. To date there has been no investigations into the effects of orally-administered ATX in the most commonly used model of ADHD, the spontaneously hypertensive rat (SHR). The aim of this study was to describe the effects of doses thought to be selective (0.15 mg/kg) and non-selective (0.3 mg/kg) for noradrenalin on behavioural measures in the SHR. Firstly, we examined the effects of acute and chronic ATX on locomotor activity including sensitisation and cross-sensitisation to amphetamine. Secondly, we measured drug effects on impulsivity using a T-maze delay discounting paradigm. We found no effect of ATX on locomotor activity and no evidence for sensitisation or cross-sensitisation. Furthermore, there were no differences in T-maze performance, indicating no effects on impulsivity at these doses. The absence of behavioural sensitisation supports previous claims of superior safety relative to psychostimulants for the doses administered. There was also no effect on impulsivity; however, we suggest that was confounded by stress specific to SHRs. Implications for future studies, behavioural assessment of SHRs and their use as a model of ADHD are discussed.Copyright © 2012 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
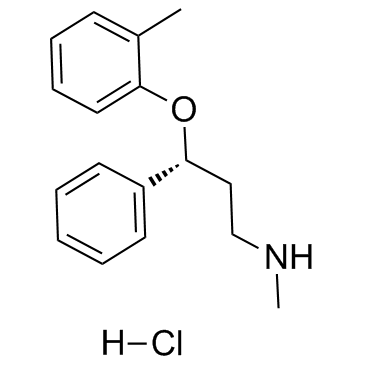 |
Atomoxetine Hydrochloride
CAS:82248-59-7 |
C17H22ClNO |
|
Methylphenidate and atomoxetine inhibit social play behavior...
2015-01-07 [J. Neurosci. 35(1) , 161-9, (2015)] |
|
Atomoxetine prevents dexamethasone-induced skeletal muscle a...
2014-12-01 [J. Pharmacol. Exp. Ther. 351(3) , 663-73, (2014)] |
|
Current pharmacotherapy of attention deficit hyperactivity d...
2013-10-01 [Drugs Today (Barc) 49(10) , 647-65, (2013)] |
|
[ADHD register: post-marketing evaluation of the benefit-ris...
2013-06-01 [Recenti Prog. Med. 104(6) , 254-61, (2013)] |
|
[Life-threatening heart failure caused by ADHD medication. F...
2012-12-01 [Lakartidningen. 109(45) , 2016-8, (2012)] |