Dioxygen reactivity of biomimetic iron-catecholate and iron-o-aminophenolate complexes of a tris(2-pyridylthio)methanido ligand: aromatic C-C bond cleavage of catecholate versus o-iminobenzosemiquinonate radical formation.
Partha Halder, Sayantan Paria, Tapan Kanti Paine
Index: Chemistry 18(37) , 11778-87, (2012)
Full Text: HTML
Abstract
An iron(III)-catecholate complex [L(1)Fe(III)(DBC)] (2) and an iron(II)-o-aminophenolate complex [L(1)Fe(II)(HAP)] (3; where L(1) = tris(2-pyridylthio)methanido anion, DBC = dianionic 3,5-di-tert-butylcatecholate, and HAP = monoanionic 4,6-di-tert-butyl-2-aminophenolate) have been synthesised from an iron(II)-acetonitrile complex [L(1)Fe(II)(CH(3)CN)(2)](ClO(4)) (1). Complex 2 reacts with dioxygen to oxidatively cleave the aromatic C-C bond of DBC giving rise to selective extradiol cleavage products. Controlled chemical or electrochemical oxidation of 2, on the other hand, forms an iron(III)-semiquinone radical complex [L(1)Fe(III)(SQ)](PF(6)) (2(ox)-PF(6); SQ = 3,5-di-tert-butylsemiquinonate). The iron(II)-o-aminophenolate complex (3) reacts with dioxygen to afford an iron(III)-o-iminosemiquinonato radical complex [L(1)Fe(III)(ISQ)](ClO(4))(3(ox)-ClO(4); ISQ = 4,6-di-tert-butyl-o-iminobenzosemiquinonato radical) via an iron(III)-o-amidophenolate intermediate species. Structural characterisations of 1, 2, 2(ox) and 3(ox) reveal the presence of a strong iron-carbon bonding interaction in all the complexes. The bond parameters of 2(ox) and 3(ox) clearly establish the radical nature of catecholate- and o-aminophenolate-derived ligand, respectively. The effect of iron-carbon bonding interaction on the dioxygen reactivity of biomimetic iron-catecholate and iron-o-aminophenolate complexes is discussed.Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
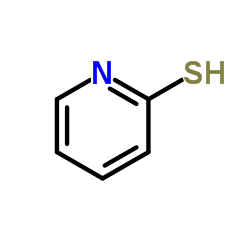 |
2-Pyridinethiol
CAS:2637-34-5 |
C5H5NS |
|
Endothelial cell-targeted pVEGF165 polyplex plays a pivotal ...
2015-01-01 [Int. J. Nanomedicine 10 , 5751-68, (2015)] |
|
Live-cell imaging of biothiols via thiol/disulfide exchange ...
1998-01-01 [Anal. Chim. Acta 849 , 57-63, (2014)] |
|
A Systematic Comparative Study of Hydrogen-Evolving Molecula...
2015-11-01 [ChemSusChem 8 , 3632-8, (2015)] |
|
Testing nucleoside analogues as inhibitors of Bacillus anthr...
2010-12-01 [Antimicrob. Agents Chemother. 54 , 5329-36, (2010)] |
|
Ag-modified Au nanocavity SERS substrates.
2009-09-14 [Phys. Chem. Chem. Phys. 11(34) , 7469-75, (2009)] |