Electron microscopy and x-ray diffraction evidence for two Z-band structural states.
Robert J Perz-Edwards, Michael K Reedy
Index: Biophys. J. 101(3) , 709-17, (2011)
Full Text: HTML
Abstract
In vertebrate muscles, Z-bands connect adjacent sarcomeres, incorporate several cell signaling proteins, and may act as strain sensors. Previous electron microscopy (EM) showed Z-bands reversibly switch between a relaxed, "small-square" structure, and an active, "basketweave" structure, but the mechanism of this transition is unknown. Here, we found the ratio of small-square to basketweave in relaxed rabbit psoas muscle varied with temperature, osmotic pressure, or ionic strength, independent of activation. By EM, the A-band and both Z-band lattice spacings varied with temperature and pressure, not ionic strength; however, the basketweave spacing was consistently 10% larger than small-square. We next sought evidence for the two Z-band structures in unfixed muscles using x-ray diffraction, which indicated two Z-reflections whose intensity ratios and spacings correspond closely to the EM measurements for small-square and basketweave if the EM spacings are adjusted for 20% shrinkage due to EM processing. We conclude that the two Z-reflections arise from the small-square and basketweave forms of the Z-band as seen by EM. Regarding the mechanism of transition during activation, the effects of Ca(2+) in the presence of force inhibitors suggested that the interconversion of Z-band forms was correlated with tropomyosin movement on actin.Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
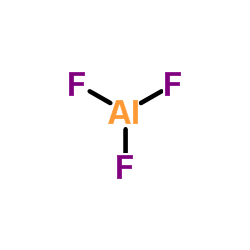 |
Aluminum fluoride
CAS:7784-18-1 |
AlF3 |
|
Comparison of the Biological Impacts of the Fluoride Compoun...
2015-09-01 [Biol. Trace Elem. Res. 167 , 84-90, (2015)] |
|
Protective effect of Spirulina and tamarind fruit pulp diet ...
2012-12-01 [Indian J. Exp. Biol. 50(12) , 897-903, (2012)] |
|
An in vitro study of the effect of aluminum and the combined...
2012-06-01 [Biol. Trace Elem. Res. 147(1-3) , 418-27, (2012)] |
|
Preparation and stabilization of aluminium trifluoroacetate ...
2012-10-07 [Dalton Trans. 41(37) , 11351-60, (2012)] |
|
Cooling-increased phospho-β-arrestin-1 and β-arrestin-1 expr...
2012-08-01 [Cryobiology 65(1) , 12-20, (2012)] |