Synthesis and antitumor activity of 5-(9-acridinylamino)anisidine derivatives.
Valeriy A Bacherikov, Jang-Yang Chang, Yi-Wen Lin, Ching-Huang Chen, Wen-Yu Pan, Huajin Dong, Rong-Zau Lee, Ting-Chao Chou, Tsann-Long Su
Index: Bioorg. Med. Chem. 13(23) , 6513-20, (2005)
Full Text: HTML
Abstract
A series of 5-(9-acridinylamino)anisidines were synthesized by condensing methoxy-substituted 1,3-phenylenediamines (10 and 11) with 9-chloroacridine derivatives to form 5-(9-acridinylamino)-m-anisidines (AMAs, 14a-e) and 5-(9-acridinylamino)-o-anisidines (AOAs, 15a-e). 5-(9-Acridinylamino)-p-anisidines (APAs, 17a-e) were synthesized by reacting 2-methoxy-5-nitroaniline (12) with 9-anilinoacridines, followed by reduction. The cytotoxic inhibition of growth of various human tumor cells in culture, inhibitory effects against topoisomerase II, and DNA interaction of these agents were studied. The structure-activity relationship studies revealed the following degree of potency: AOAs > AMAs > APAs. They also revealed that the newly synthesized derivatives bearing CONH(2)NH(2)NMe(2) and Me substituents at C4 and C5 positions of the acridine chromophore (i.e., AMA 14e, AOA 15e, and APA 17e) exhibited significant cytotoxicity against human tumor cell growth in vitro. AOA (15e) was the most potent among these derivatives, which resulted in 60% suppression of tumor volume at a dose of 20 mg/kg (Q2D x 9), intravenous injection on day 26 in nude mice bearing human breast carcinoma MX-1 xenografts.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
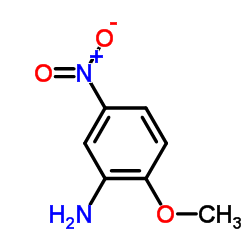 |
2-Amino-4-Nitroanisole
CAS:99-59-2 |
C7H8N2O3 |
|
Removal of multi-substituted nitroaromatic pollutants by zer...
2015-09-14 [Phys. Chem. Chem. Phys. 17 , 22072-8, (2015)] |
|
Microbial toxicity of the insensitive munitions compound, 2,...
2013-11-15 [J. Hazard. Mater. 262 , 281-7, (2013)] |
|
(Bio)transformation of 2,4-dinitroanisole (DNAN) in soils.
2016-03-05 [J. Hazard. Mater. 304 , 214-21, (2015)] |
|
5-Nitro-ortho-anisidine.
1982-04-01 [IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 27 , 133-9, (1982)] |
|
A Weibull model for the estimation of tumorigenic potency.
1993-06-01 [Biometrics 49(2) , 367-77, (1993)] |