Acute corneal melt associated with topical bromfenac use.
Pawan Prasher
Index: Eye Contact Lens 38(4) , 260-2, (2012)
Full Text: HTML
Abstract
To report a case of acute corneal melt associated with use of bromfenac ophthalmic solution.Case report.A 61-year-old man developed acute corneal melt 5 days after having combined cataract and pterygium surgery in his left eye. Postoperatively, he had been using bromfenac eyedrops four times daily along with the combination of ofloxacin and dexamethasone six times and timolol eyedrops twice daily. Ocular examination revealed the presence of asymptomatic dry eyes. He was managed conservatively with topical antibiotics, lubricants, and bandage contact lens application. The corneal melt healed completely with best-corrected visual acuity of 20/30 at 4 weeks postoperatively.The current case suggests that corneal melt can occur as a complication of inadvertent excessive use of topical bromfenac in the presence of preexisting ocular surface disorders. However, good visual outcome can be achieved by prompt conservative treatment if accurate diagnosis is made at an early stage.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
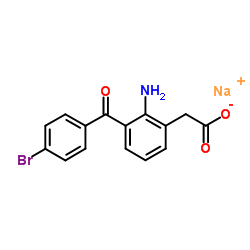 |
Bromfenac Sodium
CAS:91714-93-1 |
C15H11BrNNaO3 |
|
Multiparametric assay using HepaRG cells for predicting drug...
2015-07-02 [Toxicol. Lett. 236 , 16-24, (2015)] |
|
Efficacy of ophthalmic nonsteroidal antiinflammatory drugs i...
2009-09-01 [J. Cataract Refract. Surg. 35(9) , 1614-8, (2009)] |
|
Cyclooxygenase (COX)-inhibiting drug reduces HSV-1 reactivat...
2009-03-01 [Curr. Eye Res. 34(3) , 171-6, (2009)] |
|
Aqueous prostaglandin E(2) of cataract patients at trough ke...
2009-06-01 [Adv. Ther. 26(6) , 645-50, (2009)] |
|
Comparison of efficacy of bromfenac sodium 0.1% ophthalmic s...
2009-06-01 [J. Ocul. Pharmacol. Ther. 25(3) , 265-70, (2009)] |