Use of 13C as an indirect tag in 15N specifically labeled nucleosides. Syntheses of [8-13C-1,7,NH2-15N3]adenosine, -guanosine, and their deoxy analogues.
Anthony J Shallop, Barbara L Gaffney, Roger A Jones
Index: J. Org. Chem. 68(22) , 8657-61, (2003)
Full Text: HTML
Abstract
We have previously reported the use of a 13C tag at the C2 of 15N-multilabeled purine nucleosides to distinguish the adjacent-labeled 15N atoms from those in an untagged nucleoside. We now introduce the use of an indirect tag at the C8 of 15N7-labeled purine nucleosides. This tag allows unambiguous differentiation between a pair of 15N7-labeled purines in which only one is 13C8 labeled. Although the very small C8-N7 coupling (<1 Hz) precludes its direct detection in 1D 15N spectra, 2D 1H-15N NMR experiments display the large C8-H8 coupling (>200 Hz) because H8 is coupled to both N7 and C8. The 13C8 atom is introduced by means of a ring closure of the exocyclic amino groups of a pyrimidinone using [13C]sodium ethyl xanthate. Here, we present methods for the syntheses of [8-13C-1,7,NH2-15N3]adenosine, -guanosine, and their deoxy analogues.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
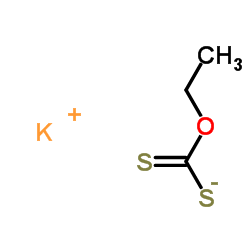 |
potassium ethylxanthate
CAS:140-89-6 |
C3H5KOS2 |
|
Time, illumination and solvent dependent stability of cadmiu...
2014-09-15 [J. Colloid. Interface Sci. 430 , 283-92, (2014)] |
|
A novel method of extracting plasmid DNA from Helicobacter s...
1998-12-01 [Helicobacter 3(4) , 269-77, (1998)] |
|
Effects of lipophilic complex formation on the disposition o...
1994-06-06 [Sci. Total Environ. 148(2-3) , 217-42, (1994)] |
|
Effects of some chelating agents on the uptake and distribut...
1994-01-01 [Pharmacol. Toxicol. 74(4-5) , 271-9, (1994)] |
|
Uptake of hydrophobic metal complexes by three freshwater al...
2009-05-01 [Environ. Sci. Technol. 43(9) , 3308-14, (2009)] |