COMU: a safer and more effective replacement for benzotriazole-based uronium coupling reagents.
Ayman El-Faham, Ramon Subirós Funosas, Rafel Prohens, Fernando Albericio
Index: Chemistry 15 , 9404-9416, (2009)
Full Text: HTML
Abstract
We describe a new family of uronium-type coupling reagents that differ in their iminium moieties and leaving groups. The presence of the morpholino group in conjunction with an oxime derivative--especially ethyl 2-cyano-2-(hydroxyimino)acetate (Oxyma)--had a marked influence on the solubilities, stabilities, and reactivities of the reagents. Finally, the new uronium salt derived from Oxyma (COMU) performed extremely well in the presence of only 1 equiv of base, thereby confirming the effect of the hydrogen bond acceptor in the reaction. COMU also showed a less hazardous safety profile than the benzotriazole-based HDMA and HDMB, which exhibited unpredictable autocatalytic decompositions. Furthermore, the Oxyma moiety contained in COMU suggests a lower risk of explosion than in the case of the benzotriazole derivatives.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
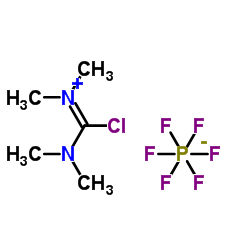 |
2,3,4-TRIFLUOROCINNAMICACID
CAS:207915-99-9 |
C5H12ClF6N2P |
|
N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate...
2008-10-01 [Bioconjug. Chem. 19 , 1968-1971, (2008)] |
|
L.A. Carpino, A. El-Faham
[J. Am. Chem. Soc. 117 , 5401, (1995)] |
|
Ayman El-Faham, et al
[European J. Org. Chem. 19 , 3641-3649, (2010)] |
|
J. Phillip Kennedy and Craig W. Lindsley
[Tetrahedron Lett. 51 , 2493-2496, (2010)] |
|
J. Habermann, H. Kunz
[J. Prakt. Chem./Chem.-Ztg. 340 , 233, (1998)] |