| Structure | Name/CAS No. | Articles |
|---|---|---|
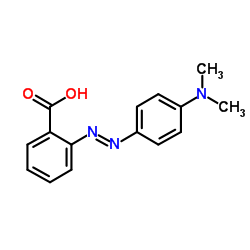 |
Methyl Red
CAS:493-52-7 |
|
![sodium 2-[4-(Dimethylamino)phenylazo]benzoate Structure](https://image.chemsrc.com/caspic/389/845-10-3.png) |
sodium 2-[4-(Dimethylamino)phenylazo]benzoate
CAS:845-10-3 |