Technological parameters of the ammonolysis of waste 1,2-dichloropropane.
Marcin Bartkowiak, Grzegorz Lewandowski, Eugeniusz Milchert
Index: J. Hazard. Mater. 106(2-3) , 107-14, (2004)
Full Text: HTML
Abstract
The ammonolysis of waste 1,2-dichloropropane (DCP) using liquid ammonia has been investigated. The influence of temperature, molar ratio of NH3/1,2-dichloropropane and reaction time was examined. The highest yield of the synthesis was achieved at a temperature of 140 degrees C, for the reaction time of 3 h and the molar ratio of NH3/1,2-dichloropropane as 20:1. Under these conditions the degree of 1,2-dichloropropane conversion amounted to 97.1 mol% and the selectivity of transformation to 1,2-diaminopropane (DAP) in relation to consumed 1,2-dichloropropane was 25.3 mol%, whereas in relation to consumed ammonia 17.9 mol%. The remaining 1,2-dichloropropane reacts to form polypropyleneamines.Copyright 2003 Elsevier B.V.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
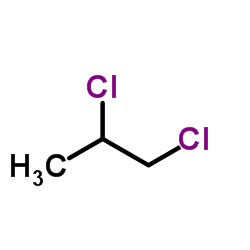 |
1,2-Dichloropropane
CAS:78-87-5 |
C3H6Cl2 |
|
Cholangiocarcinoma among offset colour proof-printing worker...
2013-07-01 [Occup. Environ. Med. 70(7) , 508-10, (2013)] |
|
Inhalation exposure in Drosophila mutagenesis assays: experi...
1991-02-01 [Mutat. Res. 252(1) , 17-33, (1991)] |
|
Distribution of 1,3-dichloropropene and 1,2-dichloropropane ...
1991-10-01 [Bull. Environ. Contam. Toxicol. 47(4) , 572-9, (1991)] |
|
Experimental and theoretical studies on formation and degrad...
2006-03-01 [Chemosphere 63(1) , 165-70, (2006)] |
|
Fraction of electrons consumed in electron acceptor reductio...
1999-09-01 [Appl. Environ. Microbiol. 65(9) , 4049-56, (1999)] |