| Structure | Name/CAS No. | Articles |
|---|---|---|
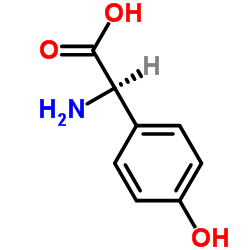 |
(D)-(-)-α-4-hydroxyphenyl glycine
CAS:22818-40-2 |
|
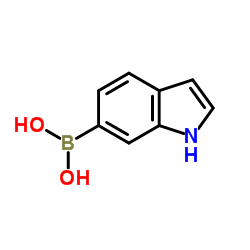 |
Indole-6-boronic acid
CAS:147621-18-9 |
|
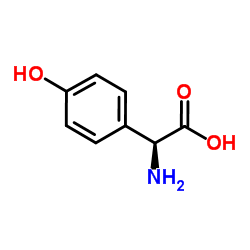 |
oxfenicine
CAS:32462-30-9 |