Inversion of configuration during hydrolysis of alpha-1,4-galacturonidic linkage by three Aspergillus polygalacturonases.
P Biely, J Benen, K Heinrichová, H C Kester, J Visser
Index: FEBS Lett. 382(3) , 249-55, (1996)
Full Text: HTML
Abstract
Endopolygalacturonases I and II (PGI and PGII) of Aspergillus niger and an exopolygalacturonase (ExoPG) of A. tubingensis were investigated to reveal the stereochemistry of their hydrolytic action. Reduced pentagalacturonic acid (pentaGalU-ol) and reduced trigalacturonic acid (triGalU-ol) were used as non-reducing substrates for the enzymes. The configuration of the reducing ends in the products formed in D2O reaction mixtures was followed by 1H-NMR spectroscopy. It has been unambiguously established that primary cleavage of pentaGalU-ol by both PGI and PGII leads to diGalU-ol and the beta-anomer of triGalUA. The primary products of hydrolysis of triGalUA-ol by ExoPG were diGal-ol and the beta-anomer of GalUA. Thus, all three Aspergillus polygalacturonases belong to the so-called inverting glycanases, i.e. they utilize the single displacement mechanism of hydrolysis of the glycosidic linkage.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
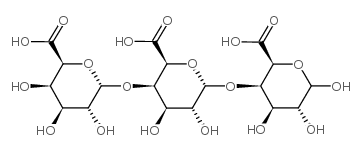 |
Trigalacturonic Acid
CAS:6037-45-2 |
C18H26O19 |
|
Molecular characterization of a thermophilic endo-polygalact...
2014-12-31 [J. Agric. Food Chem. 62(52) , 12686-94, (2015)] |
|
Oligosaccharide formation during commercial pear juice proce...
2016-08-01 [Food Chem. 204 , 84-93, (2016)] |
|
A Novel Acid-Stable Endo-Polygalacturonase from Penicillium ...
2016-06-28 [J. Microbiol. Biotechnol. 26 , 989-98, (2016)] |
|
Purification and Properties of Polygalacturonase Produced by...
2013-01-01 [Enzyme Res. 2013 , 438645, (2013)] |
|
Horizontal Gene Transfer of Pectinases from Bacteria Precede...
2016-01-01 [Sci. Rep. 6 , 26388, (2016)] |