Modeling of reaction steps relevant to deoxyuridylate (dUMP) enzymatic methylation and thymidylate synthase mechanism-based inhibition.
A Leś, L Adamowicz, W Rode
Index: J. Biomol. Struct. Dyn. 15(4) , 703-15, (1998)
Full Text: HTML
Abstract
Theoretical quantum mechanical ab initio Hartree-Fock calculations on molecular systems, modeling processes related to the specificity of thymidylate synthase inactivation are reported. We considered several steps of the methylation of the substrate dUMP and 4- or 5-mono- and 4,5-bisubstituted dUMP analogs, as well. The following reactions were modeled: the cysteine residue (Cys198 in the L.casei enzyme) nucleophilic attack on the substrate and the substrate C(5)-H proton abstraction. The substrate was modeled by the 1-methyluracil molecule and its structural analogs. The cysteine Cys198 residue was modeled by the methylmercaptane molecule. The substrate-enzyme binary complex was modeled by the 1-methyl-5,6-dihydro-6-thiomethyl-uracil (P1) molecule. The present theoretical calculations suggest that the cysteine nucleophilic attack on the substrate may result in the SH-group addition to the pyrimidine C(5)=C(6) bond in the course of a weakly exothermic reaction. The formerly presumed enolate carbanion appeared to be weakly stable or unstable and it can readily split into the thiol and pyrimidine residues. The s2-thio- (P2) and s2,4-dithio- (P3) substrate analogs should form stable thiolate anions after cysteine residue attachment to the C(6) position of the pyrimidine ring. Studies of the deformed P1 molecule interacting with a water molecule bound to the pyrimidine C(4)=O carbonyl residue allow a suggestion that this water molecule may be directly involved in the C(5)-H proton abstraction and may serve as a proton transmitter between the substrate and the proton acceptor residue, possibly located on the cofactor N10-nitrogen. Interaction of the pyrimidine C(4)=O group, or its modification, with the N5,10-methylenetetrahydrofolate N(10) nitrogen atom is suggested as an additional factor influencing the inhibition process.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
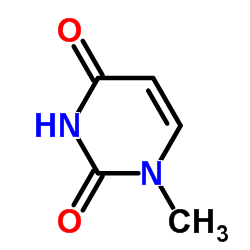 |
methyluracil
CAS:615-77-0 |
C5H6N2O2 |
|
Orotic acid decarboxylation in water and nonpolar solvents: ...
2009-09-15 [Biochemistry 48(36) , 8738-45, (2009)] |
|
Thermodynamic, kinetic and structural studies on the ternary...
2000-04-01 [J. Inorg. Biochem. 79(1-4) , 129-38, (2000)] |
|
Theoretical and spectroscopic study of infrared spectra of h...
2008-04-28 [J. Chem. Phys. 128(16) , 164506, (2008)] |
|
Pulse radiolytic studies of radicals produced from methylate...
1988-05-01 [Radiat. Res. 114(2) , 207-14, (1988)] |
|
Assessment of hepatic tissue blood flow with 133Xe clearance...
1981-01-01 [Int. J. Nucl. Med. Biol. 8(1) , 77-84, (1981)] |