Rimonabant dimorphism and its pressure-temperature phase diagram: a delicate case of overall monotropic behavior.
Marc-Antoine Perrin, Michel Bauer, Maria Barrio, Josep-Lluís Tamarit, René Céolin, Ivo B Rietveld
Index: J. Pharm. Sci. 102(7) , 2311-21, (2013)
Full Text: HTML
Abstract
Crystalline polymorphism occurs frequently in the solid state of active pharmaceutical ingredients, and this is problematic for the development of a suitable dose form. Rimonabant, an active pharmaceutical ingredient developed by Sanofi and discontinued because of side effects, exhibits dimorphism; both solid forms have nearly the same melting temperatures, melting enthalpies, and specific volumes. Although the problem may well be academic from an industrial point of view, the present case demonstrates the usefulness of constructing pressure-temperature phase diagrams by direct measurement as well as by topological approach. The system is overall monotropic and form II is the more stable solid form. Interestingly, the more stable form does not possess any hydrogen bonds, whereas the less stable one does.Copyright © 2013 Wiley Periodicals, Inc.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
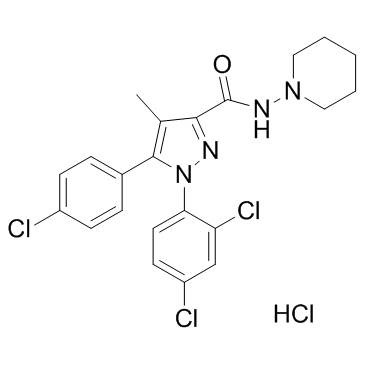 |
SR 141716A
CAS:158681-13-1 |
C22H22Cl4N4O |
|
Obesity drug therapy.
2013-09-01 [Minerva Endocrinol. 38(3) , 245-54, (2013)] |
|
Anandamide-CB1 receptor signaling contributes to postnatal e...
2013-04-10 [J. Neurosci. 33(15) , 6350-66, (2013)] |
|
Reduced food intake is the major contributor to the protecti...
2013-09-01 [Yonsei Med. J. 54(5) , 1127-36, (2013)] |
|
Peripherally restricted CB1 receptor blockers.
2013-09-01 [Bioorg. Med. Chem. Lett. 23(17) , 4751-60, (2013)] |
|
Role of adiponectin in the metabolic effects of cannabinoid ...
2014-02-15 [Am. J. Physiol. Endocrinol. Metab. 306(4) , E457-68, (2014)] |