Lack of development of tolerance to anticonvulsant effects of two excitatory amino acid antagonists, CGP [corrected] 37849 and CGP 39551 in genetically epilepsy-prone rats.
G De Sarro, A De Sarro, D Ammendola, S Patel
Index: Brain Res. 734(1-2) , 91-7, (1996)
Full Text: HTML
Abstract
Two selective excitatory amino acid antagonists, DL-(E)-2-amino-4-methyl- 5-phosphono-3-pentenoic acid (CGP 37849) and its carboxyethylester (CGP 39551), were studied against audiogenic seizures in genetically epilepsy-prone rats following oral administration. Acute administration of CGP 37849 attenuated the clonic and tonic phases of the audiogenic seizures (109 dB, 12-16 kHz) 120 min after pretreatment (ED50 19.7 and 11.2 mumol kg-1, respectively). Similarly, CGP 39551 attenuated the clonic and tonic phases of audiogenic seizures 120 min after acute treatment with ED50 values of 17.2 and 8.8 mumol kg-1, respectively. For chronic studies animals were treated orally once daily (at 10 h) for 4 weeks with CGP 37849 (20 or 40 mumol kg-1) or CGP 39551 (15 or 30 mumol kg-1). In order to assess anticonvulsant activity, rats were subjected to auditory stimulation 120 min after drug administration on days 1, 3 and 5 and then every 3 or 4 days. Following 2 and 4 weeks of repeated drug administration with CGP 37849 (20 and 40 mumol kg-1) the ED50 values against clonic and tonic seizures were not significantly different from those observed following an acute administration. Similarly, 2 and 4 weeks after repeated treatment CGP 39551 (15 and 30 mumol kg-1) the ED50 values against clonic and tonic seizures were not significantly different from those observed following an acute administration. There was no significant difference between the ED50 values following either acute or repeated treatment of the two excitatory amino acid antagonists suggesting a lack of development tolerance. The duration of anticonvulsant activity observed between 0.5 and 24 h following administration of CGP 37849- and CGP 39551 was similar in acute and chronic treatment. The effects of CGP 37849 and CGP 39551 on motor behaviour was also evaluated following acute and repeated treatment by a rotarod apparatus 110 min following drug administration. The TD50 values for CGP 37849 and CGP 39551-induced impairment of locomotor performance recorded 2 or 4 weeks of repeated administration were not significantly different from those observed following an acute administration. The TD50 values for CGP 37849- and CGP 39551-induced impairment of locomotor performance were 87.6 and 70.8 mumol kg-1 i.p. respectively following 2 weeks treatment and 92.9 and 76.9 mumol kg-1 i.p. respectively following 4 weeks treatment. The doses of CGP 37849 and CGP 39551 required to elicit motor impairment were at least an order of magnitude above required for anticonvulsant activity. Since these compounds showed anticonvulsant properties after oral administration and lack of development of tolerance after repeated treatment, a potential use for antiepileptic therapy in man is suggested.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
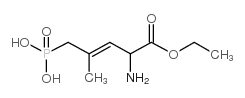 |
CGP 39551
CAS:127910-32-1 |
C8H16NO5P |
|
Competitive NMDA receptor antagonists and agonists: effects ...
2004-01-01 [Pol. J. Pharmacol. 56(1) , 59-66, (2004)] |
|
NMDA receptor antagonists acting at the glycineB site in rat...
1999-01-01 [J. Neural Transm. Gen. Sect. 106(11-12) , 1189-204, (1999)] |
|
Effects of competitive NMDA receptor antagonists on excitato...
1999-01-01 [Fundam. Clin. Pharmacol. 13(1) , 67-74, (1999)] |
|
Effect of CGP39551 administration on the kindling of ethanol...
2002-09-01 [Psychopharmacology 163(2) , 157-65, (2002)] |
|
Chronic pre-explant blockade of the NMDA receptor affects su...
1997-03-17 [Brain Res. Dev. Brain Res. 99(1) , 112-7, (1997)] |