Regulation of the retention factor for weak acids in micellar electrokinetic chromatography with cationic surfactant via variation of the chloride concentration.
Irma Orentaitė, Audrius Maruška, Ute Pyell
Index: Electrophoresis 32(5) , 604-13, (2011)
Full Text: HTML
Abstract
For tetradecyltrimethylammonium bromide in boric acid/borate or acetic acid/acetate buffer and NaCl or CaCl₂ as the added salt, it is investigated whether the retention behaviour of weak acids in MEKC with cationic surfactant can be modelled by assuming for the deprotonated species predominantly electrostatic interaction with the micelles acting as a pseudostationary ion-exchanger. The retention of (partially) charged solutes by oppositely charged micelles is analyzed by applying the classical theory of IEC (plotting lg k against lg(c(Cl⁻)) assuming a fixed concentration of ion-exchange sites. When plotting the absolute slopes of the regression lines against the absolute effective charge numbers of the analytes, correlation coefficients of 0.968-0.998 were obtained. It is shown that the dependence of the retention factor on the concentration of chloride (the competing ion) in the separation electrolyte and on the degree of dissociation of the analyte corresponds to what would be expected for mixed-mode retention (hydrophobic and ion-exchange interaction) on a pseudostationary ion-exchanger.Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
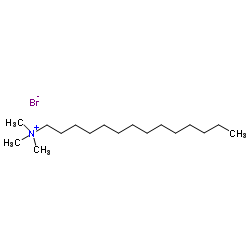 |
Tetradecyltrimethylammonium bromide
CAS:1119-97-7 |
C17H38BrN |
|
A Solid Phase Vibrational Circular Dichroism Study of Polype...
2015-12-01 [Chirality 27 , 965-72, (2015)] |
|
Correlation of 3-mercaptopropionic acid induced seizures and...
2014-06-16 [Eur. J. Pharm. Sci. 57 , 25-33, (2014)] |
|
Determination of pyruvate and lactate as potential biomarker...
2015-06-01 [Electrophoresis 36 , 1244-50, (2015)] |
|
Small molecule inhibitors of aggregation indicate that amylo...
2007-04-06 [J. Biol. Chem. 282 , 10311-24, (2007)] |
|
Photoreversible polymer-surfactant micelles using the molecu...
2013-03-12 [Langmuir 29(10) , 3188-94, (2013)] |